[edit]
Scoping Report on Hospital and Health Care Acquisition of COVID-19 and its Control
This paper has drawn on evidence available up to 28 June 2020. Further evidence on this topic is constantly published and DELVE may return to this topic in the future. This independent overview of the science has been provided in good faith by subject experts. DELVE and the Royal Society accept no legal liability for decisions made based on this evidence.
Summary
- The focus of this report is on SARS-CoV-2 infection acquired in hospital. It is timed to inform decisions about the opening up of hospitals and other healthcare settings for non-COVID-19 activities and NHS plans for winter and future waves of COVID-19.
- Transmission of COVID-19 in hospitals and social care settings in patients, residents and staff has been recognised as an important feature of the COVID-19 epidemic throughout the world; efforts to prevent infections have had varying success. Transmissions early in the epidemic reflected evolving understanding of the disease (e.g., the risk of asymptomatic infection and role of masks in source control) and limited access to testing and personal protective equipment.
- Infections in hospital have important implications for infection outcomes (hospitalised patients and some staff are at higher risk), workforce planning (healthcare workers being unable to work during peak pressure periods), and amplification of community transmission (through discharge of infectious patients and transmission to families and other contacts from healthcare workers and patients).
- Using publicly available data, we estimate that at least 10% (95% confidence interval: 4-15%) of all COVID-19 infections in England were among patient-facing healthcare workers and resident-facing social care workers during the period from 26th April to 7th June 2020. An estimated further 1% of infections in this period were acquired by inpatients in hospital, with an additional 6% of all infections among care home residents. This does not consider secondary cases arising from hospital acquired infections elsewhere.
- Although surveillance systems and large-scale hospital-based studies have recently been set up, there remain gaps in availability of surveillance data on hospital-acquired infections, particularly of healthcare workers (including agency staff) and in nursing homes; important questions remain unanswered, including about the impact on Black, Asian and minority ethnic (BAME) health and social care workers.
- At present, there is no single organisation with clear oversight of hospital-related infection surveillance, monitoring and response. Data obtained from surveillance, monitoring and outbreak investigations need to feed into epidemiological and modelling research, including to evaluate interventions. There remain significant opportunities to link epidemiological and phylogenetic datasets to improve the ability to identify and respond to changing epidemic dynamics.
- In recent weeks, there have been significant improvements reported in data collection and prevention of hospital acquired infection, but the data are not yet publicly available to assess the impact of changes and these are urgently needed.
- The report considers what further actions are needed to build comprehensive surveillance and infection control systems, with awareness that this would require further resources and expert support to the hospital, public health and care sectors. It sets out a suggested framework for effective centralised surveillance and monitoring of hospital acquired infections, linked to rapid infection control responses with sharing of best practice, coordinated through local teams. In the medium-term, we envisage an ambitious and comprehensive approach to prevention of infection transmitted through respiratory droplets and aerosol routes in hospitals, of the breadth and scale successfully implemented for methicillin-resistant Staphylococcus aureus (MRSA).
Citation
(2020), Scoping Report on Hospital and Health Care Acquisition of COVID-19 and its Control. DELVE Report No. 3. Published 06 July 2020. Available from https://rs-delve.github.io/reports/2020/07/06/nosocomial-scoping-report.html [pdf].
Table of Contents
- Problem statement
- Background
- Current sources and gaps in understanding of hospital-related COVID-19 transmission
- Epidemiological evidence of hospital acquired transmission5
- Successful infection control practices for COVID-19
- Summary considerations
1. Problem statement
Despite recent declines in COVID-19 cases there remains a significant risk of future epidemic waves, which may overlap with other pressures on the NHS and care system (particularly influenza, but also other infections) over the winter. There is an urgent need to safely open up hospitals to provide non-COVID-19 inpatient and outpatient care. There has been substantial heterogeneity in hospital acquired infection rates, in trust-level infection control responses and in policies across the devolved national administrations. Systematic data collection over the course of the epidemic thus far has been insufficient to identify the extent, sources and risks for hospital acquired transmission and to allow effective targeted outbreak response and infection control. Substantial recent investments have, however, been made to improve surveillance and control, but comprehensive, accessible national data are currently limited. For example, the absolute number of healthcare workers affected is unknown, as is the time and place of acquisition of infection. This lack of information is of particular importance in the case of Black, Asian and minority ethnic (BAME) healthcare workers amid widespread concerns that their mortality from COVID-19 exceeds their representation in the work force. Similarly, there has been only limited exploration of the extent to which hospital acquired transmission amplifies wider community spread, including to and from institutional settings such as care homes, especially when there was a lack of testing capacity within the NHS and social care.
This report provides an outline of the considerations in building a robust surveillance and response system to prevent future hospital acquired COVID-19 outbreaks. It will:
-
consider key areas to address in the hospital response;
-
highlight evidence gaps in understanding hospital-related transmission;
-
identify broad areas for systematic interventions.
2. Background
Hospitals are high-risk sites for COVID-19 transmission because:
-
physical distancing is not always possible, leading to unavoidable close contacts between healthcare workers and patients, and contact between health workers on wards, non-patient and rest areas;
-
they can be sites of inadvertent mixing of COVID-19-infected and uninfected patients, for example, due to asymptomatic cases that are unrecognised as infectious;
-
many hospitalised patients and some staff are more vulnerable to COVID-19 infection and its complications – due to higher prevalence of both comorbid conditions and other risk factors (e.g., socioeconomic status, ethnicity);
-
healthcare workers and patients may transmit the infection into the community, including to their families, contacts and to highly vulnerable populations in care homes.
Prevention of hospital-acquired infections – among both staff and patients – could substantially impact epidemic transmission and help limit resurgence. Healthcare worker infections are important both because of the impact on their personal health, the risk of transmission to patients, and because they lead to loss of key healthcare providers for prolonged periods, both when infected and when required to quarantine, often at the time of maximum strain on the workforce. Hospital-acquired patient infections pose a greater clinical concern than community-acquired ones because complication rates for those hospitalised for other reasons are higher than in the wider population. Since hospitals connect a wide range of households and institutions within their communities, hospital-acquired infections can seed infections in care homes and other residential institutions, their surrounding communities and within the hospital itself (see Figure 1).
The primary focus of this report will be on transmission occurring within the hospital setting (acknowledging that identifying such transmissions has challenges). We recognise that these transmissions can have wider impact, due to the movement of individuals between hospitals and care homes, shared staff housing and other community settings. While we will not cover policies for these extra-hospital movements (and related infections) in detail, we will consider hospital practices that might amplify wider community transmission.
Despite the absence of systematic data on hospital-acquired infections, there is little doubt that they have been substantial. A recent modelling study estimated that 20% of inpatient infections, and up to 89% of healthcare worker infections, were hospital acquired.1 In a major London teaching hospital, 15% of inpatients with COVID-19 from 2nd March to 12th April 2020 had a hospital-acquired infection, with a case fatality rate of 36%.2 The Dynamic COVID-19 Clinical Information Network (CO-CIN) report to SAGE and NERVTAG, which captures data on those testing positive within healthcare settings, estimated that at the end of March, 10% of inpatients with COVID-19 had acquired their infection in hospital.3
Comprehensive data on the scale of healthcare worker infections are limited. However, healthcare worker COVID-19 prevalence was estimated to be nearly six times higher than in the general population in England based on PCR testing (1.87% (1.07%-3.02%) tested positive compared to 0.32% (0.25%-0.44%) in the general population).4 In one London hospital, over a one-month period during the peak of the epidemic in March and April 2020, 45% of tested clinical staff were antibody-positive and 21% nucleic acid test positive at some point; most had no symptoms [5]. Such asymptomatic or mildly symptomatic healthcare workers are particularly concerning because both groups may pose an unrecognised transmission risk to others in the hospital and in the community. Two surveys in UK hospitals have found 57% (17/30, 95%CI: 37-75%) and 81% (34/42, 95%CI: 66-91%) of PCR-positive workers to have few or no symptoms.5 6 Mildly symptomatic healthcare workers may continue working for the first few days of illness; at one Seattle hospital 61% of infected healthcare workers with mild symptoms continued to work.7 Limiting infection control precautions to those with identified compatible symptoms will therefore be insufficient.
Using publicly available data, we estimate that at least 10% (95% CI: 4-15%) of all COVID-19 infections in England between 26th April and 7th June were among patient-facing healthcare workers and resident-facing social care workers (Annex 1). However, we emphasise that we cannot determine from the ONS data source the proportion of infections that were occupationally acquired. During the same period, we estimate that at least 1% of all COVID-19 infections were inpatients who acquired their infection in hospital, and that 6% (95% CI: 4-8%) of all COVID-19 infections were care home residents. The wide range of confidence intervals presented partly reflect the limited publicly available data. All our calculations are based on plausible, conservative estimates. They do not consider the unknown fraction of secondary cases arising from healthcare-acquired infections or the associated mortality, which has been concentrated in hospital and care home cases (Figure 2).
Hospital outbreaks (two or more linked infections) remain ongoing even as the number of cases is dropping nationwide; 31 were reported in England between June 1st and 7th and 21 in the following week.8 9 Data from CO-CIN highlight that the absolute number of patients with definite hospital-acquired infection (symptoms arose ≥14 days after admission) began to rise from mid-March, peaking around 1st April. However, the proportion of all patients with COVID-19 who had hospital-acquired infection did not peak until 1st May (Figure 3). Large clusters of infections have been publicly reported in three acute care hospitals (Addenbrookes, Western General, Weston General); the Addenbrookes outbreak involved multiple clusters of infection, including both patients and healthcare workers, although the mechanisms and directions of transmissions are not yet well understood.10 This combination of larger and smaller outbreaks highlights the heterogeneity in COVID-19 hospital-acquired infections and may reflect differences in infection prevention and control measures implemented by trusts. However, it is clear that most hospital-acquired COVID-19, both of patients and healthcare workers, can be prevented if optimal infection prevention and control practices are followed, as exemplified by both international and domestic experiences, even in the more difficult to control care home setting.11 12 13 14 15 16 17
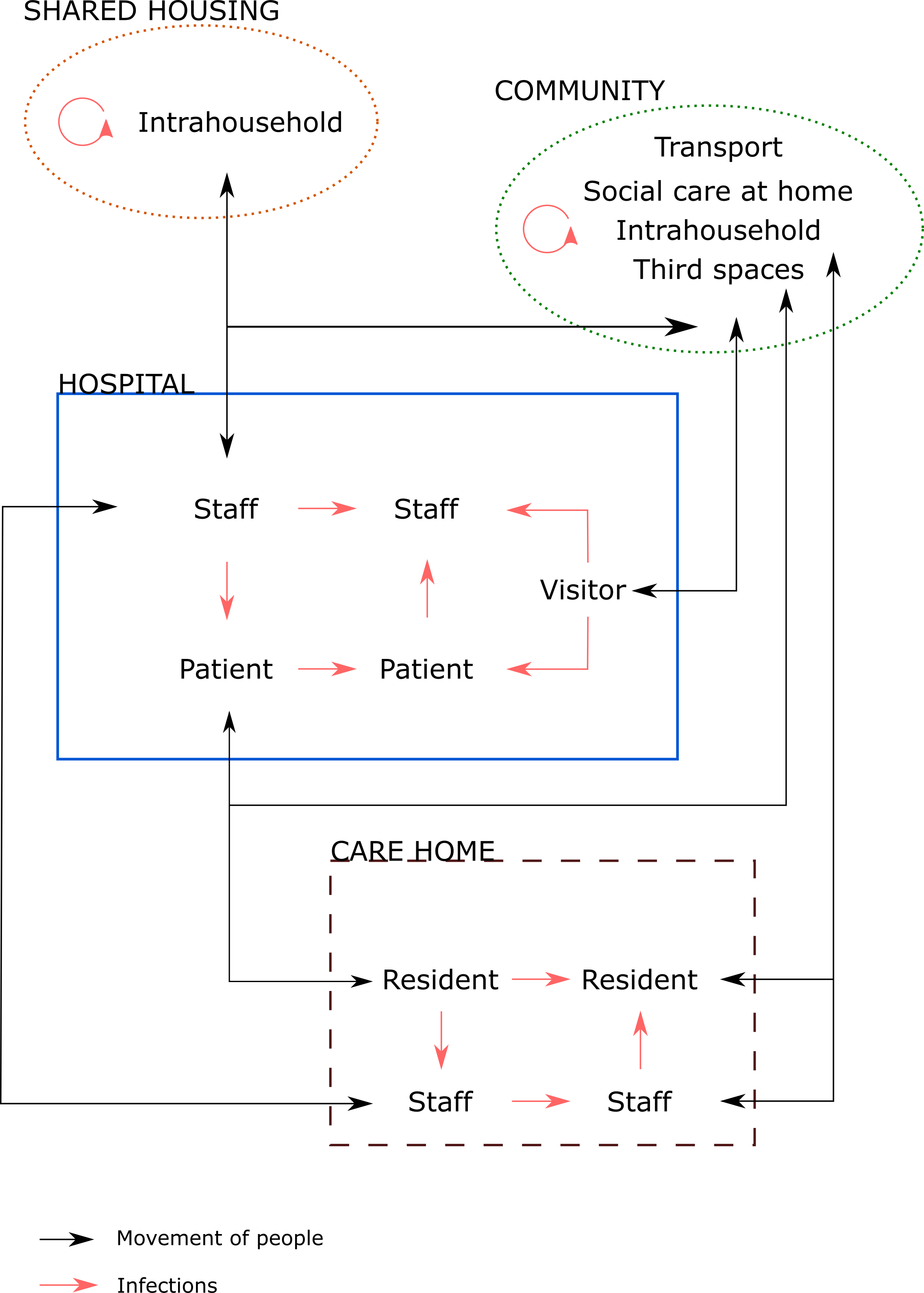
Figure 1. Schematic of the flow of individuals and possible routes of infection in and around hospital settings
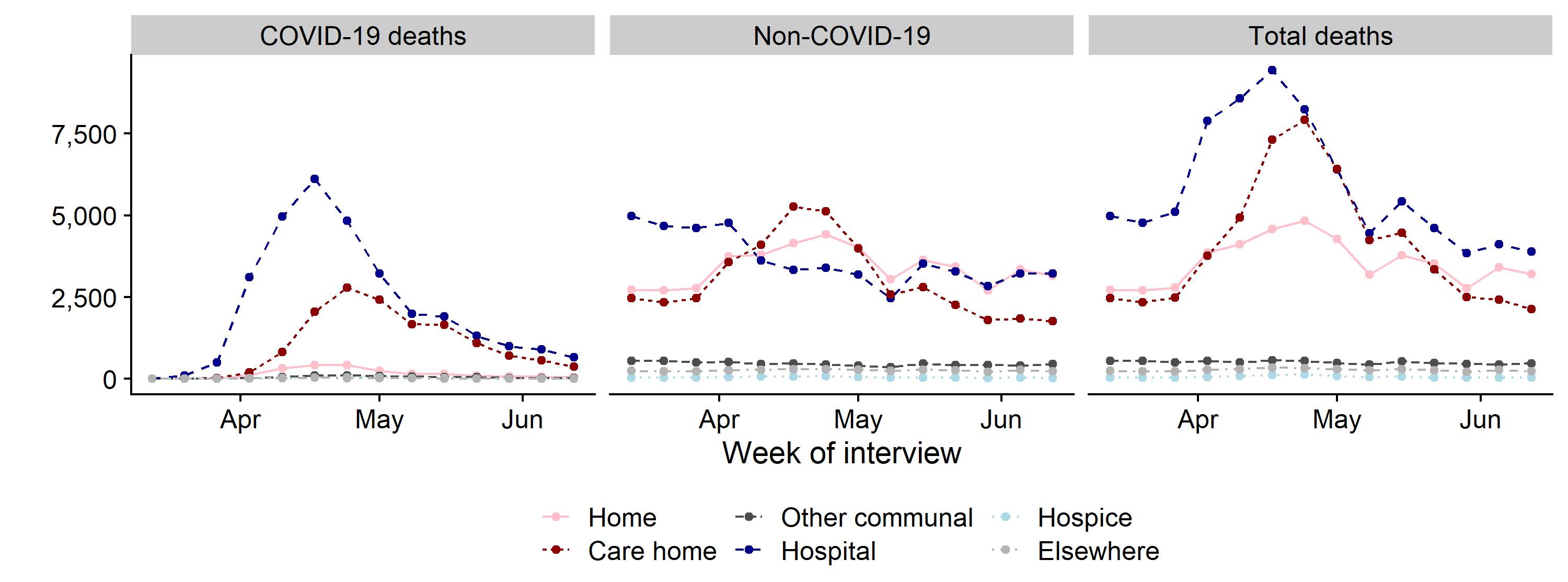
Figure 2. Weekly mortality in England and Wales by place of occurrence
Source: Adapted from Deaths registered weekly in England and Wales, provisional.18
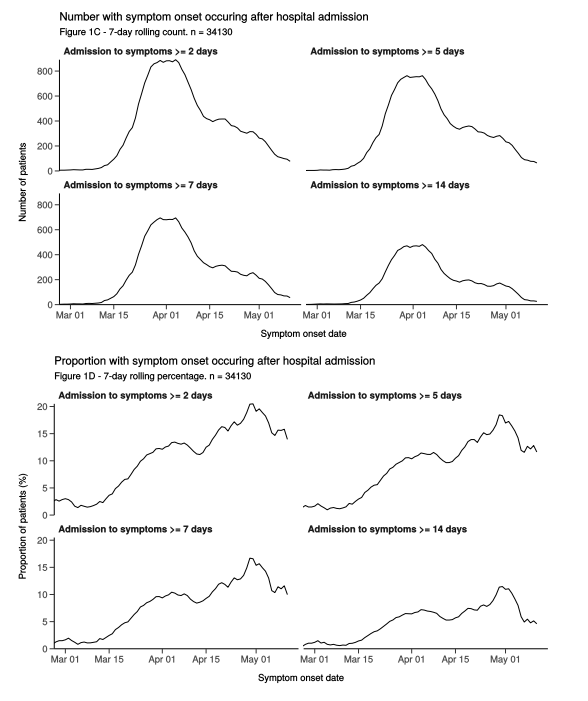
Source: Dynamic CO-CIN report to SAGE and NERVTAG, 21 May 202019
Figure 3. Number and proportion of patients with COVID-19 with symptom onset after hospital admission
3. Current sources and gaps in understanding of hospital-related COVID-19 transmission
In order to address and minimise the risks of hospital transmission, there is a need for better evidence on how it arises (key transmission routes), who it affects (time, person and place), and how it is propagated onwards (transmission mechanism). We recognise that important steps have been taken during the epidemic to date and the challenges of updating systems in the midst of a pandemic. Initial problems with PPE supply and limited test availability, which restricted PCR testing for staff, have been resolved. Steps have included a review of approaches to understanding how COVID-19 spreads in hospitals early in the epidemic.20 Recommendations for changes to environmental procedures in hospitals were made to the Scientific Advisory Group for Emergencies (SAGE) in mid-May,21 masks in healthcare settings were mandated from 15 June 2020,22 and a recent letter to all Trusts from NHS England highlighted best practice for infection reporting, and outbreak management.23 A great deal of information on COVID-19 in hospitals has also been collected, including several nationwide efforts. These include:
-
COVID-19 Clinical Information Network (CO-CIN).24 This network captures around half of all hospitalisations with COVID-19, adding hand-collected demographics and hospital episode data to positive COVID-19 test results. This is resource intensive and misses some cases but generates a rich dataset. CO-CIN has been reporting to SAGE regularly since 1st April.25
-
SIREN study. Public Health England (PHE) is conducting a study to evaluate the level of antibodies in 100,000 healthcare workers and determine the level of protective immunity past infection may offer (Susan Hopkins, personal communication). While longitudinal in design, SIREN uses a convenience sample and thus will be subject to selection bias.
-
NHS Situation Reports. Since late May, the NHS has been compiling a weekly national SitRep on the COVID-19 situation; these are not currently publicly available.
-
PHE dashboard. PHE is developing a dashboard that links several routine datasets to estimate the number and proportion of hospital acquired COVID-19 infections at NHS Trust level (Susan Hopkins, personal communication). These datasets include Pillar 1 COVID-19 laboratory test results (Second Generation Surveillance System (SGSS)), hospital episodes statistics (HES) and Emergency Care discharge summaries. This project uses existing routinely available datasets. However, data processing and identification of hospital-acquired cases requires careful assumptions and there are points where information can be lost. The dashboard does not include detailed epidemiological data and is unable to capture healthcare worker infections.
-
Office for National Statistics (ONS) survey.26 The ONS has been conducting regular population-based surveys since late May, including capturing information on whether participants are healthcare workers. Non-response for these surveys is substantial (over 60% for the pilot study), which may lead to bias even after weighting procedures, and information on hospital acquisition is not available. However, this dataset provides regular, well-powered evidence on trends in prevalence.
-
COVID Symptom Study smartphone application. Developed by a private company (ZOE) and researchers at Kings College London, this app allows users to track their symptoms and pool information for analysis. While this produces a non-random sample of participants, data from this study has been used to estimate that healthcare workers in the UK and US had over 11 times the hazard of having tested positive for COVID-19, and 3.4 time the hazard after adjusting for likelihood of having tested.27
-
COVID-19 in care homes (VIVALDI) study.28 This cohort study aims to observe what proportion of 11,500 care home staff and residents have been previously infected with SARS-CoV-2 at baseline, and through serial antibody and PCR testing and data linkage with NHS and other sources, estimate how many become infected and the duration of the antibody response. Initial high-level results were published on 3rd July.29
While these various sources provide substantial data, each of them has limitations, and triangulation between them has not been systematically conducted to date. Moreover, data from these systems are not normally publicly available, although some (e.g., ONS, CO-CIN) are available on request through a governed process. As a result, there are a number of key information gaps that limit our ability to understand and tackle hospital acquired infections:
-
Patient infections. While the identities (and thus socio-demographics and comorbidities) of individuals testing positive are often captured, the time and place of onset (and thus possible transmission source) is not documented in a consistent or coordinated manner. This information, in conjunction with systematic surveillance data from residential care settings (which is currently also lacking) can help determine where improved infection prevention and control measures are required. A short standardised reporting system for each positive result (similar to systems previously used for methicillin-resistant Staphylococcus aureus and Clostridioides difficile) could help with this along with root-cause analysis, as recently requested by NHS England;30 Trust-level reporting of probable and definite healthcare-associated COVID-19 infections, is required since COVID-19 is a notifiable disease; however these data are not yet publicly available.
-
Healthcare worker infections. Although SIREN, a large-scale healthcare worker survey to measure prevalence and incidence has been established recently, data on healthcare worker acquisition have been substantially more limited than on patients. Comprehensive information will be needed including time of onset, time and place (hospital setting, other institution, home) of possible acquisition, relevant exposures, role/occupation, risk factors, comorbidities and socio-demographics. Addressing concerns about excess morbidity and mortality in BAME healthcare workers requires data such as these. While testing of healthcare worker and social care staff is being conducted, alongside widespread PCR and antibody testing (Pillars 1,2 and 431), comprehensive data sampling frames, analyses and results are not currently available.
-
Population-level staffing. Agency and other mobile staff may have been implicated in several institutional COVID-19 outbreaks, however, very little is known about how outbreaks relate to staff absences and the use of temporary staff (which itself may facilitate transmission between healthcare settings). Comprehensive data on staff sickness and other absences, and temporary staff arrival and mobility, would allow identification of patterns predictive of outbreak risk and targeted prevention interventions, and enable better workplace planning.
-
Hospital outbreak investigations. Effective interventions to stop transmission within hospitals require systematic investigations directly linked to recommendations for infection prevention and control changes. Such investigations are the responsibility of Trust Directors of Infection Prevention and Control (DIPC) and guidance has recently been strengthened.32 These changes could be monitored using local or regional assistance and peer learning from hospitals that have successfully eliminated outbreaks. While greater involvement of local PHE Health Protection Teams (led by consultants in communicable disease control (CCDC)) and Local Authority Directors of Public Health (DsPH) has commenced, there is no comprehensive national database on the nature or outcomes of these investigations. Such a database would allow an evidence base to be built, from which learning and interventions can be optimised.
-
Data linkage. Effective interpretation and use of all the above information rely on joined-up data. This includes linkages between: i) hospitals and their local community, including related care institutions; and ii) epidemiological and phylogenetic data to understand the characteristics of individuals with linked infections and make valid inferences about likely transmission events.
-
Systematic PHE involvement. The current situation, where PHE and local public health specialists can only investigate hospital acquired outbreaks when invited to do so by the local hospital trust is not conducive to full understanding of the national situation.
In establishing the Joint Biosecurity Centre (JBC), several of these gaps may be addressed by bringing together new and existing initiatives, including Test and Trace and outbreak investigations, into a comprehensive, centralised surveillance system capturing all infections acquired by patients and healthcare workers and any onward transmission. Data collected in the areas listed above will be synergistic in providing benefits. The availability of the above data will enable key questions to be answered, either directly or via modelling based on these parameters as inputs. Key questions include, but are not limited to:
-
Does patient acquisition/outcome differ by occupation, clinical setting, ethnicity and social deprivation?
-
What are the relative contributions of transmission pathways (patient to patient, patient to staff, staff to staff, etc (see Figure 1))?
-
What are the relative contributions of transmission locations (wards, theatres, bathrooms, shared staff spaces) and modes (direct droplet, aerosol, fomites)?
-
How does staff movement between hospital wards in the same hospital, or between hospitals, outpatient clinics and care homes amplify disease spread?
-
What is the national, regional and local fraction of healthcare worker infection that can be attributed to transmission at work (from patients or other healthcare workers), as opposed to community-acquisition, and does this fraction change with levels of infection in the community or other risk factors (e.g., ethnicity)?
-
What fraction of community-acquired cases are likely seeded from: (1) healthcare workers; (2) patients discharged to care facilities; (3) patients discharged home?
-
Where do hospital interventions need to be focused to minimise the risk of outbreaks?
-
What specific interventions work to bring hospital acquired outbreaks under control?
-
How well are hospitals able to implement recommendations and what additional support do they need to achieve them?
-
Are there differences in how the devolved nations of the UK and other countries have experienced and responded to hospital transmission that would inform future policy?
Determining the answers to these questions will require a clear mandate and resources. It will be challenging because of the need to distinguish between the potential explanations for apparent transmission chains linked to hospitals. The route of transmission for healthcare workers and patients can be difficult to ascertain with certainty because of the variable incubation period and clinical course of the disease, and because it is difficult to discriminate between exposure to hospital and community environments as the source, making it difficult to ascertain transmission dynamics. Molecular strain typing may be useful to help answer these (noting important caveats about inferring transmission and its direction), but its utility is predicated on timely linkage to epidemiology data (time, person, place), which is not currently widely available. It is likely that some of these questions, and data collection and modelling for them, will need to be prioritised, but the ordering of such priorities is likely to shift as the epidemic progresses.
4. Epidemiological evidence of hospital acquired transmission
Internationally, hospital-acquired COVID-19 has been linked to a number of inadequate infection prevention and control practices:
-
Failure to use personal protective equipment (PPE) appropriate for personal encounters, such as face masks, eye protection, gowns and gloves, either because of the lack of availability of appropriate equipment or lack of recognition of an infected patient or healthcare worker;
-
Inadequate hand washing and respiratory hygiene;
-
Failure to properly disinfect surfaces and maintain overall environmental hygiene and ventilation;
-
Failure to eliminate the potential for fomites to transmit infection within or carry infection into or out of hospitals;
-
Lack of escalation of infection prevention and control practices during patient encounters involving aerosol generation or exposure to mucosal surfaces and fluids;
-
Lack of appropriate physical distancing;
-
Failure to separate infected from non-infected patients;
-
Rotation of staff between infected and uninfected patient locations or between hospitals;
-
Inadequate testing and tracing capacity.
Much of the evidence for these modes of transmission is inferred from reports of hospital acquired transmission and one well-documented transmission in a care home earlier in the pandemic.
-
Nine separate clusters of infection involving 35 healthcare workers and family members were reported from one Wuhan hospital when COVID-19 infections were occurring but unrecognised; they were attributed to lack of use of PPE in the hospital and large staff gatherings.33
-
Hospital acquired infection of 80 healthcare workers and 39 patients in one South African hospital was attributed to lack of the use of isolation and droplet and contact precautions, insufficient hand washing, delayed recognition of a COVID-19-infected patient, transferring infected patients between different wards and inadequate surface disinfection.34
-
From neurosurgery departments in 107 hospitals in Hubei province, 120 doctors and nurses were infected.35 The source of infection was reported to be a work colleague (38% of infections), an infected patient (29%), an infected family member (4%) or unknown (29%). Transmission was attributed to inadequate PPE and unrecognised contagious patients or colleagues.
-
In a Bavarian maternity centre, 36 staff members were infected.36 The initial source was found to be a midwife who had recently returned from a ski trip. She became ill with a respiratory illness, later shown to be COVID-19 with several transmission chains following. The midwife attended a staff meeting at the clinic on the day that she became ill. The transmission chain was attributed to a failure to recognise a case of COVID-19 infection, staff meetings and eating in a canteen. Social distancing in break rooms and the staff canteen, and universal mask use were put in place with no further outbreaks at the clinic.
-
A febrile healthcare worker reported to work at a paediatric dialysis unit in Germany, resulting in the infection of 28 healthcare workers, 13 patients and 7 patient companions.37 The outbreak was attributed to work attendance of a symptomatic infected worker, lack of physical distancing and lack of use of appropriate PPE.
-
In the geriatric unit of a French hospital, a patient admitted with fever and respiratory symptoms that was not recognised to be COVID-19 led to 4 other patients and a healthcare worker on the same unit becoming infected.38 The outbreak was attributed to a super spreader event enabled by the lack of droplet and contact precautions, lack of universal use of enhanced infection prevention and control measures for all patients, and lack of timely COVID-19 diagnosis or suspicion.
-
In a Seattle care home, a symptomatic worker introduced COVID-19 into the facility.39 The nursing home began using enhanced infection prevention and control practices two days after the introduction of the infection, but before its recognition. Staff were screened for symptoms and symptomatic residents were cared for using COVID-19-appropriate PPE. However, there was a high rate of presymptomatic disease, allowing spread of infection to several facility wings. A total of 57 residents (64% of all residents) and 26 staff (18% of all staff) were infected. Infected staff included nurses, as well as physical therapists, food delivery staff and janitorial staff, many of whom worked in multiple facility wings. Eleven of the infected residents were hospitalised and 15 died. The lack of recognition of presymptomatic spread of infection, and the assumption that asymptomatic persons were not contagious, was the main factor that was linked to spread of infection in the facility. Universal infection prevention and control measures regardless of symptoms aborted the outbreak.
-
In an acute hospital in London, a review of 435 inpatient cases of COVID-19 between 2nd March and 12th April 2020 found that 66 (15%) were probably or definitely hospital acquired.40 There was evidence of patient-to-patient transmission between cases in the same bay, but the likely source could not be identified for most cases and might have been staff members, other patients or visitors with unrecognised infection. A comprehensive infection prevention and control (IPC) response was mounted, which included expansion of PPE use and cohorting of suspected cases; the number and proportion of hospital acquired cases subsequently fell. There was also evidence of high infection rates in healthcare workers in the same hospital: a cohort of 181 healthcare workers enrolled in late March and early April found 45% were antibody positive by one-month follow-up.41
These reports documenting hospital acquired transmission highlight that infection control practices that may be in place to prevent transmission of non-respiratory pathogens like hepatitis B, methicillin-resistant Staphylococcus aureus (MRSA) and enteric infections will be insufficient to prevent SARS-CoV-2 which appears to transmit predominantly through the respiratory droplet route.
5. Successful infection control practices for COVID-19
There is much publicly available guidance on specific infection prevention and control practices. There is also evidence that hospital acquired infection has been controlled in some cases.42 43 Guided by our analysis of this literature, and evidence of infection prevention and control practices used in the UK and elsewhere (44 45 46 47 48 49 50 51 52 and Annex 2), we summarise the following important measures that have been reported to reduce hospital-acquired transmission, organised according to transmission routes shown in Figure 1.
-
Minimise risk of importation into hospitals
a. Consistent and reasonable limits on visitors (varying by local epidemic state)
b. Minimise agency/multi-site staffing and staff movements between sites (including hospitals, care homes and other institutions) as practical
c. Entry screening, or self-certification, of staff, patients and visitors for signs and symptoms of COVID-19 infection53
d. Maximise use of telemedicine and remote consultations
- Minimise risk of transmission within hospitals
- Aerosol/droplet transmission
-
Requirements to wear surgical/cloth masking by staff, visitors and patients wherever feasible, for source control; in addition to staff masking for wearer-protection in specific high-risk settings54
-
Organise hospitals into COVID-19 stratified “hot” and “cold” zones, or entire hospitals into “hot” and “cold” hospitals if feasible
iii. Cohort staff to limit physical overlap and movement between zones if possible, i.e., “bubbling”
iv. Limits on communal activities for healthcare workers (break rooms, cafeteria) and maintenance of physical distancing
v. Move Multidisciplinary Team and other meetings online as much as possible
vi. Maximise use of remote consultations for outpatients
vii. Maintain effective airflow handling systems (acknowledging building infrastructure limits)
viii. Physical distancing, especially in likely hotspots (e.g., waiting rooms, triage locations, A&E, corridors, lifts, staff lounges, canteens or cafeterias)
-
b. Contact transmission
i. Repeated frequent handwashing for everyone present in facility
ii. Ensuring access to appropriate personal protective equipment for all staff
iii. Training on donning and doffing personal protective equipment (e.g., masks, gowns, gloves, visors), and mandating staff use as appropriate
iv. Reduced sharing of equipment (e.g., computer keyboards)
v. Expanded and improved environmental cleaning procedures (including exploring novel methods such as UV light, antiviral surfaces, optimum surfaces)
- Aerosol/droplet transmission
-
Minimise risk of outbreak occurrence and expansion within hospital
a. Active clinical surveillance of patients for the development of COVID-19 infection
b. Prompt isolation, testing, investigation and contact tracing of infected healthcare workers
c. Prompt quarantine of healthcare workers and patients with significant exposure to known cases, and self-monitoring for those with lesser exposure
d. Rapid and standardised outbreak investigations and reporting, including root-cause analysis
e. Provision of sick leave without penalty or prejudice based on symptoms (including contract staff)
-
Minimise risk of exportation from hospitals
a. Testing/quarantining of patients being discharged to own home or institutional care
The simultaneous use of overlapping infection control measures has made it difficult to evaluate the importance of each measure individually. However, multiple coordinated precautions are likely to be required, reinforced by comprehensive staff training linked to continuous professional development. Strong leadership is needed at a high level across the healthcare service to ensure that these are applied and monitored effectively and consistently to minimise hospital acquired infection and prevent onward transmission to the community and thus further pressures on hospitals.
6. Summary considerations
To minimise and control hospital-acquired COVID-19 infections, we recommend continuing and enhanced investment in standardised, timely, nationwide hospital surveillance systems to track and analyse trends and undertake rapid, locally led, outbreak control. Many of the ideas below are being developed within the COVID-19 response by the JBC and PHE among others; our aim is to highlight the urgent need for their systematic and joint implementation, and transparent rapid access to data. We envisage an ambitious and comprehensive approach to prevention of infection transmitted through respiratory droplets and aerosols in hospitals, of the kind successfully implemented for MRSA.55
-
Rapid identification of COVID-19 cases within hospitals. Establish a standardised risk-based protocol for testing individuals within hospitals, both for COVID-19 and other key respiratory pathogens such as influenza, including consistent intensity, breadth of coverage and speed of results turnaround. This protocol would need to reach beyond existing systems for testing patients to cover hospital employees (including staff without patient-facing roles), students and volunteers.
-
Centralised surveillance and monitoring of COVID-19 infections acquired within hospitals. Building on existing reporting systems, standardised COVID-19 case reporting would allow the rapid identification of hospitals with certain or probable outbreaks and cases linked to them, with the potential for external oversight (e.g. the Care Quality Commission). Direct surveillance might be supported by using routinely available data, such as on staff absences which can also inform workforce planning decisions, and processed results such as phylogenetic data. Reports should be publicly available, and include case numbers and reductions over time, as part of hospital performance metrics.
-
Connected COVID-19 data systems across community, care institutions and hospitals. Information on local community incidence and links to institutional settings, including long-term care facilities, may empower hospitals to assess the risk of importing and exporting cases, and take appropriate preventative measures.
-
Standardised, tiered infection prevention and control guidelines. These guidelines should vary by risk level, based on closely monitored infection levels; much of this already exists, but requires integration with the other considerations in this section.
-
Regional or local outbreak investigations for COVID-19. A minimum standard for outbreak investigations should be set centrally, with external oversight (e.g., the JBC, PHE) and a standardised reporting structure. While the preferred approach to outbreak management should be through empowerment of Trust or hospital-level structures (e.g., Directors of Infection Prevention and Control), but may require central resources. Outbreak investigations should include support from successful local/regional peer institutions, including PHE and Directors of Public Health. Report findings should lead to enforceable, externally monitored recommendations of interventions to reduce hospital transmission, with mandated executive-level responsibility for implementation. All hospital-acquired infections should be linked to existing Test and Trace systems.
-
Research platform. Data obtained from surveillance, monitoring and outbreak investigations should feed into epidemiological and modelling research and evaluation. This research is needed to evaluate which interventions are cost-effective and feasible in preventing hospital and social care transmission in the long-term.
Technical Appendices
The following are materials prepared by individual members of DELVE as inputs into this report.
- NOS-TD1: Estimating COVID-19 infections of patient-facing healthcare workers and resident-facing social care workers
Prepared for the DELVE Initiative by
- NOS-TD2: Management of Nosocomial (Hospital-Acquired) Infections of SARS-CoV2
Prepared for the DELVE Initiative by
Footnotes and References
-
Evans, S., Agnew, E., Vynnycky, E. and Robotham, JV. (2020) The impact of testing and infection prevention and control strategies on within-hospital transmission dynamics of COVID-19 in English hospitals. medRxiv. doi: 10.1101/2020.05.12.20095562 ↩
-
Rickman HM, Rampling T, Shaw K, Martinez-Garcia G, Hail L, Coen P, et al. Nosocomial transmission of COVID-19: a retrospective study of 66 hospital-acquired cases in a London teaching hospital. Clin Infect Dis. 2020. doi: https://doi.org/10.1093/cid/ciaa816 ↩
-
SAGE (2020) Dynamic CO-CIN report to SAGE and NERVTAG (1 April 2020). Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/886424/s0096-co-cin-report-010420-sage22.pdf ↩
-
Office for National Statistics (2020) Coronavirus (COVID-19) Infection Survey pilot: 5 June 2020: Office for National Statistics. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/coronaviruscovid19infectionsurveypilot/5june2020/pdf ↩
-
Houlihan, C., Vora, N., Byrne, T., Lewer, D., Heaney, J., Moore, DA. et al. (2020) SARS-CoV-2 virus and antibodies in front-line Health Care Workers in an acute hospital in London: preliminary results from a longitudinal study. medRxiv. doi: 10.1101/2020.06.08.20120584v1 ↩
-
Rivett, L., Sridhar, S., Sparkes, D., Routledge, M., Jones, NK., Forrest, S. et al. (2020) Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. Elife. doi: 10.7554/eLife.58728. PubMed PMID: 32392129 ↩
-
Chow, EJ., Schwartz, NG., Tobolowsky, FA., Zacks, RLT., Huntington-Frazier, M., Reddy, SC. et al. (2020) Symptom Screening at Illness Onset of Health Care Personnel With SARS-CoV-2 Infection in King County, Washington. JAMA. 2020. doi:10.1001/jama.2020.6637 ↩
-
Public Health England (2020) Weekly Coronavirus Disease 2019 (COVID-19) Surveillance Report: Summary of COVID-19 surveillance systems. Week 24 2020. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/891721/Weekly_COVID19_Surveillance_Report_-_week_24.pdf ↩
-
Public Health England (2020) Weekly Coronavirus Disease 2019 (COVID-19) Surveillance Report: Summary of COVID-19 surveillance systems. Week 25 2020. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/893216/Weekly_COVID19_Surveillance_Report_w25.pdf ↩
-
Meredith, LW., Hamilton, WL., Warne, B., Houldcroft, CJ., Hosmillo, M., Jahun, A. et al. (2020) Rapid implementation of real-time SARS-CoV-2 sequencing to investigate healthcare-associated COVID-19 infections. medRxiv. doi: 10.1101/2020.05.08.20095687 ↩
-
Jones, NK., Rivett, L., Sparkes, D., Forrest, S., Sridhar, S., Young, J. et al. (2020) Effective control of SARS-CoV-2 transmission between healthcare workers during a period of diminished community prevalence of COVID-19. eLife. doi: 10.7554/eLife.59391 ↩
-
Gawande A. (2020) Amid the Coronavirus Crisis, a Regimen for Reëntry. The New Yorker. 2020 3/13/2020:1-15. Available at: https://www.newyorker.com/science/medical-dispatch/amid-the-coronavirus-crisis-a-regimen-for-reentry ↩
-
Gawande, A. (2020) Keeping the Coronavirus from Infecting Health-Care Workers: What Singapore’s and Hong Kong’s success is teaching us about the pandemic. The New Yorker. 2020 3/21/2020:1-7. Available at: https://www.newyorker.com/news/news-desk/keeping-the-coronavirus-from-infecting-health-care-workers ↩
-
Dora, AV., Winnett, A., Jatt, LP., Davar, K., Watanabe, M., Sohn, L. et al. (2020) Universal and Serial Laboratory Testing for SARS-CoV-2 at a Long-Term Care Skilled Nursing Facility for Veterans
- Los Angeles, California, 2020. MMWR Morbidity and Mortality Weekly Reports. doi: 10.15585/mmwr.mm6921e1. PubMed PMID: 32463809
-
Wee, LE., Conceicao, EP., Sim, XYJ., Aung, MK., Tan, KY., Wong, HM. et al. (2020) Minimizing intra-hospital transmission of COVID-19: the role of social distancing. J Hosp Infect. 2020. doi: 10.1016/j.jhin.2020.04.016. PubMed PMID: 32294511 ↩
-
Cheng, VCC., Wong, S-C., Chuang, VWM., So, SYC., Chen, JHK., Sridhar, S. et al. (2020) Absence of nosocomial transmission of coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 in the pre-pandemic phase in Hong Kong. Am J Infect Control. 2020. doi: 10.1016/j.ajic.2020.05.018. PubMed PMID: 32461068 ↩
-
Cheng, VCC., Wong, S-C., Chen, JHK., Yip, CCY., Chuang, VWM., Tsang, OTY. et al (2020). Escalating infection control response to the rapidly evolving epidemiology of the coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 in Hong Kong. Infect Control Hosp Epidemiol. doi: 10.1017/ice.2020.58. PubMed PMID: 32131908 ↩
-
Office for National Statistics (2020) Deaths registered weekly in England and Wales up to week ending 12 June 2020, provisional. Available from: https://www.ons.gov.uk/file?uri=%2fpeoplepopulationandcommunity%2fbirthsdeathsandmarriages%2fdeaths%2fdatasets%2fweeklyprovisionalfiguresondeathsregisteredinenglandandwales%2f2020/publishedweek242020.xlsx ↩
-
SAGE (2020) Dynamic CO-CIN report to SAGE and NERVTAG (21 May 2020). Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/893440/S0428_Dynamic_CO-CIN_Report_to_SAGE_and_NERVTAG__1_.pdf ↩
-
Powis, S. (2020) Nosocomial Transmission of Coronavirus: Research and management. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/892032/S0091_Nosocomial_Transmission_of_Coronavirus.pdf ↩
-
SAGE Environmental and Modelling Group (2020). Possible additional interventions to address hospital transmission risks of SARS-CoV-2 2020. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/892014/S0344_Possible_additional_interventions_to_address_hospital_transmission_risks.pdf ↩
-
UK Government Department of Health and Social Care (2020) Face masks and coverings to be worn by all NHS hospital staff and visitors. Published 5 June. Available from: https://www.gov.uk/government/news/face-masks-and-coverings-to-be-worn-by-all-nhs-hospital-staff-and-visitors ↩
-
May, R., Powis, S., Issar, P., Philip, P. (2020) Healthcare associated COVID-19 infections – further action. Available from: https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/06/Healthcare-associated-COVID-19-infections--further-action-24-June-2020.pdf ↩
-
Docherty, AB., Harrison, EM., Green, CA., Hardwick, HE., Pius, R., Norman, L. (2020) et al. Features of 16,749 hospitalised UK patients with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol. medRxiv. doi: 10.1101/2020.04.23.20076042 ↩
-
SAGE (2020) Dynamic CO-CIN report to SAGE and NERVTAG (1 April 2020). Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/886424/s0096-co-cin-report-010420-sage22.pdf ↩
-
Office for National Statistics (2020) Coronavirus (COVID-19) Infection Survey pilot: 5 June 2020: Office for National Statistics. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/coronaviruscovid19infectionsurveypilot/5june2020/pdf ↩
-
Nguyen, LH., Drew, DA., Joshi, AD., Guo, C-G., Ma, W., Mehta, RS. et al. (2020) Risk of COVID-19 among frontline healthcare workers and the general community: a prospective cohort study. medRxiv. doi: 10.1101/2020.04.29.20084111 ↩
-
Shallcross L. (2020) COVID-19 in care homes (VIVALDI). ISRCTN Registry. Available from: https://doi.org/10.1186/ISRCTN14447421 ↩
-
Office for National Statistics (2020) Impact of coronavirus in care homes in England: 26 May to 19 June 2020. Published 3 July. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/impactofcoronavirusincarehomesinenglandvivaldi/26mayto19june2020 ↩
-
May, R., Powis, S., Issar, P., Philip, P. (2020) Healthcare associated COVID-19 infections – further action. Available from: https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/06/Healthcare-associated-COVID-19-infections--further-action-24-June-2020.pdf ↩
-
There are five pillars to COVID-19 testing in England; these are: (1) NHS swab testing for medical need and, where possible, the most critical key workers; (2) Mass-swab testing for critical key workers; (3) Mass-antibody testing for immunity; (4) Population surveillance testing; (5) Scaling up national diagnostics . ↩
-
May, R., Powis, S., Issar, P., Philip, P. (2020) Healthcare associated COVID-19 infections – further action. Available from: https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/06/Healthcare-associated-COVID-19-infections--further-action-24-June-2020.pdf ↩
-
Wang, X., Zhou, Q., He, Y., Liu, L., Ma, X., Wei, X. et al. (2020) Nosocomial outbreak of COVID-19 pneumonia in Wuhan, China. Eur Respir J. doi: 10.1183/13993003.00544-2020. PubMed PMID: 32366488 ↩
-
Lessells, R., Moosa, Y., de Oliveira, T. (2020) Report into a nosocomial outbreak of coronavirus disease 2019 (COVID‐19) at Netcare St. Augustine’s Hospital. Durban, South Africa: 2020 5/15/2020. Available at: https://www.krisp.org.za/news.php?id=421 ↩
-
Wang, Q., Huang, X., Bai, Y., Wang, X., Wang, H., Hu, X. et al. (2020) Epidemiological characteristics of COVID-19 in medical staff members of neurosurgery departments in Hubei province: A multicentre descriptive study. medRxiv. doi: 10.1101/2020.04.20.20064899 ↩
-
Kabesch, M., Roth, S., Brandstetter, S., Hausler, S., Juraschko, E., Weigl, M. et al. (2020) Successful containment of COVID-19 outbreak in a large maternity and perinatal center while continuing clinical service. Pediatr Allergy Immunol. 2020. doi: 10.1111/pai.13265. PubMed PMID: 32319131 ↩
-
Schwierzeck, V., Konig, JC., Kuhn, J., Mellmann, A., Correa-Martinez, CL., Omran, H. et al. (2020) First reported nosocomial outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a pediatric dialysis unit. Clin Infect Dis. 2020. doi: 10.1093/cid/ciaa491. PubMed PMID: 32337584 ↩
-
Vanhems, P., Saadatian-Elahi, M., Chuzeville, M., Marion, E., Favrelle, L., Hilliquin, D. et al. (2020) Rapid nosocomial spread of SARS-CoV-2 in a French geriatric unit. Infect Control Hosp Epidemiol. 41(7):866-7. Epub 2020/03/30. doi: 10.1017/ice.2020.99 ↩
-
Arons, MM., Hatfield, KM., Reddy, SC., Kimball, A., James, A., Jacobs, JR. et al. (2020) Presymptomatic SARS-CoV-2 Infections and Transmission in a Skilled Nursing Facility. N Engl J Med. 2020. doi: 10.1056/NEJMoa2008457 ↩
-
Rickman HM, Rampling T, Shaw K, Martinez-Garcia G, Hail L, Coen P, et al. Nosocomial transmission of COVID-19: a retrospective study of 66 hospital-acquired cases in a London teaching hospital. Clin Infect Dis. 2020. doi: https://doi.org/10.1093/cid/ciaa816 ↩
-
Houlihan, C., Vora, N., Byrne, T., Lewer, D., Heaney, J., Moore, DA. et al. (2020) SARS-CoV-2 virus and antibodies in front-line Health Care Workers in an acute hospital in London: preliminary results from a longitudinal study. medRxiv. doi: 10.1101/2020.06.08.20120584v1 ↩
-
Wu, Z. and McGoogan, JM. (2020) Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-42 ↩
-
World Health Organization (2020) Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19). Geneva: WHO, 2020. Available from: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf ↩
-
Rivett, L., Sridhar, S., Sparkes, D., Routledge, M., Jones, NK., Forrest, S. et al. (2020) Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. Elife. doi: 10.7554/eLife.58728. PubMed PMID: 32392129 ↩
-
Gawande A. (2020) Amid the Coronavirus Crisis, a Regimen for Reëntry. The New Yorker. 2020 3/13/2020:1-15. Available at: https://www.newyorker.com/science/medical-dispatch/amid-the-coronavirus-crisis-a-regimen-for-reentry ↩
-
Gawande, A. (2020) Keeping the Coronavirus from Infecting Health-Care Workers: What Singapore’s and Hong Kong’s success is teaching us about the pandemic. The New Yorker. 2020 3/21/2020:1-7. Available at: https://www.newyorker.com/news/news-desk/keeping-the-coronavirus-from-infecting-health-care-workers ↩
-
Kabesch, M., Roth, S., Brandstetter, S., Hausler, S., Juraschko, E., Weigl, M. et al. (2020) Successful containment of COVID-19 outbreak in a large maternity and perinatal center while continuing clinical service. Pediatr Allergy Immunol. 2020. doi: 10.1111/pai.13265. PubMed PMID: 32319131 ↩
-
Schwierzeck, V., Konig, JC., Kuhn, J., Mellmann, A., Correa-Martinez, CL., Omran, H. et al. (2020) First reported nosocomial outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a pediatric dialysis unit. Clin Infect Dis. 2020. doi: 10.1093/cid/ciaa491. PubMed PMID: 32337584 ↩
-
Htun, HL., Lim, DW., Kyaw, WM., Loh, W-NJ., Lee, LT., Ang, B. et al. (2020) Responding to the COVID-19 outbreak in Singapore: Staff Protection and Staff Temperature and Sickness Surveillance Systems. Clin Infect Dis. 2020. doi: 10.1093/cid/ciaa468. PubMed PMID: 32315026 ↩
-
Kim, YJ., Jeong, YJ., Kim, SH., Kim, YJ., Lee, SY., Kim, TY. et al. (2020) Preparedness for COVID-19 infection prevention in Korea: a single-centre experience. J Hosp Infect. 2020. doi: 10.1016/j.jhin.2020.04.018 ↩
-
Klompas, M., Morris, CA., Sinclair, J., Pearson, M., Shenoy, ES. (2020) Universal Masking in Hospitals in the Covid-19 Era. N Engl J Med. 2020. doi: 10.1056/NEJMp2006372. PubMed PMID: 32237672 ↩
-
Wee, LE., Conceicao, EP., Sim, XYJ., Aung, MK., Tan, KY., Wong, HM. et al. (2020) Minimising intra-hospital transmission of COVID-19: the role of social distancing. J Hosp Infect. 2020. doi: 10.1016/j.jhin.2020.04.016. PubMed PMID: 32294511 ↩
-
While potentially useful, entry-screening like all testing procedures, are open to generating false-positive and false-negative results. One aspect of this is the variation in sensitivity and specificity of tests across the duration of individuals’ infections and how the specimen is collected. A second aspect is that test result intreptation should take into account current epidemic circumstances, since positive and negative predictive values vary with population prevalence. ↩
-
DELVE: Data Evaluation and Learning for Viral Epidemics (2020) Face Masks for the General Public. Published 4 May. Report No 1. Available from: https://rs-delve.github.io/reports/2020/05/04/face-masks-for-the-general-public.html ↩
-
Duerden, B., Fry, C., Johnson, AP., Wilcox, MH. (2015) The control of methicillin-resistant Staphylococcus aureus blood stream infections in England. Open Forum Infectious Diseases. 2015;2(2):ofv035. doi: 10.1093/ofid/ofv013 ↩