[edit]
Test, Trace, Isolate
This work was carried out rapidly in May 2020 so that an early version could inform discussions at SAGE. It may be revisited as further scientific evidence becomes available.
Summary
When effectively implemented at scale, Test, Trace, Isolate (TTI) can contribute to controlling the UK COVID-19 epidemic, but only as part of a wider package of public health interventions, including physical and social distancing, control of infection procedures, outbreak investigation and control. TTI is most effective in breaking chains of transmission, and reducing the effective reproductive number ($R_e$), when there is maximum: (i) speed, i.e., quick turn-around of both index case testing and contact tracing (and testing); (ii) compliance, i.e., a high proportion of people in each chain are willing and able to follow guidance; and (iii) coverage, i.e., identification of most chains through integration of consistent case data and real-time, high-precision population surveillance. Each of these three aspects of TTI needs careful attention, as do the trade-offs implicit in choices of how precisely to implement TTI in terms of who to test, trace and isolate, and when to do so.
Based on our modelling work, we confirm that social distancing and self-isolation of symptomatic individuals and quarantine of their household contacts has a substantial impact on the number of new infections generated by each index case. We further show that adding contact tracing for extra-household contacts of confirmed cases to this broader package of interventions reduces the number of new infections otherwise generated by 5-15%. The upper end of this range represents scenarios where the overall test and trace period for contacts has been reduced from five days to three days. Furthermore, the level of compliance with TTI guidance strongly affects its usefulness, as there are many steps in the TTI system at which cases and contacts can be lost. Phone-based apps may be able to increase TTI speed and compliance but is likely to be an adjunct to a manual TTI system. Both incentives and clear messaging relating to TTI participation and compliance with isolation measures are also likely to be needed to maximize TTI’s effectiveness.
TTI and surveillance systems are mutually beneficial, since TTI can capture important data on index cases and contacts, while surveillance can provide indications of who/where to target for testing, even when they are not part of an identified transmission chain. Finally, TTI will require substantial coordination across a wide range of organizations, including central and local government departments, PHE, the NHS and business groups. In particular, local integration of systems is likely to maximize ability to conduct the agile, locally differentiated outbreak management that may be needed as the epidemic evolves.
The Royal Society’s News Page gives an overview of the report: https://royalsociety.org/news/2020/05/success-of-test-trace-and-isolate-programmes-depends-on-speed-compliance-and-monitoring
Citation
(2020), Test, Trace, Isolate. DELVE Report No. 2. Published 27 May 2020. Available from https://rs-delve.github.io/reports/2020/05/27/test-trace-isolate.html [pdf].
Table of Contents
- Key points
- Test Trace Isolate
- The role of TTI in COVID-19 response
- Factors that influence optimal performance of a TTI system
- Increasing speed of testing and tracing
- Maximizing population participation and compliance with TTI guidance
- Increasing epidemic coverage of TTI
- Managing TTI capacity constraints
- Effective TTI within a broader epidemic response
- Next steps
- Technical Appendices
- Footnotes and References
Key points
-
The role of TTI in COVID-19 response. At time of writing, it seems likely that the UK’s focus in the short- to medium-term will be on containing the COVID-19 epidemic and driving down case numbers, while balancing policies to reduce transmission alongside policies to manage the wider health and economic impacts of the epidemic. Test, Trace, Isolate (TTI), defined as the chained process of testing index individuals with symptoms or known contact with past cases, monitoring contacts of these index cases and potentially limiting their interactions (through isolation and quarantine) can play an important role in controlling COVID-19. In combination with other measures, TTI has been comprehensively implemented early in epidemics in several countries who have controlled their epidemics, and maintained when case numbers increased.
-
Factors that influence optimal performance of a TTI system. COVID-19, like any infectious disease epidemic, is a collection of transmission chains. Modelling by several teams, including new analysis presented here, in addition to experiences with previous epidemics, highlights that TTI for COVID-19 can be effective at breaking chains of transmission when: (i) there is quick turn-around between testing index cases and tracing (and testing) contacts; (ii) a high proportion of people in each chain are identified and willing and able to comply with TTI guidance; and (iii) a high proportion of transmission chains are identified through real-time, high-precision surveillance (i.e., regular, quasi-random nationwide diagnostic testing). It is notable that countries with successful COVID-19 control strategies integrated TTI within a wider framework of measures, and were effective in all three of these areas. Each of these three aspects of TTI therefore needs careful attention.
-
Increasing speed of testing and tracing. The short serial interval (average time between index case and infected contact becoming infectious, estimated at 5-6 days) and high level of likely pre-symptomatic transmission of COVID-19 means that speed is of the essence in using TTI to break infection chains. When there are delays in getting tests for index cases and tracing contacts of proven cases, secondary cases are often not found until they themselves have been infectious for some time. Shortening the time taken to test and to trace individuals makes a substantial impact on the relative effectiveness of TTI: our simulation model finds that reducing the overall turnaround time from five days to three leads to 60% greater reduction in $R_e$ due to contact tracing of extra-household contacts. Since the impact of a TTI system on secondary infections is dominated by the large impact of immediate isolation of symptomatic cases and their household contacts, the absolute effect of speeding up testing and tracing is modest. Testing and tracing can be sped up using existing infrastructure and experiences from optimising care for other health conditions (e.g., Tuberculosis, sexually transmitted infections). Policy choices on overall TTI capacity are critical and affect decisions on how to be utilise TTI. As capacity is approached, TTI speed will be strongly affected, and bringing more people into the system (e.g., tracing contacts before index test results are known, testing non-symptomatic contacts) can create trade-offs in terms of resource requirements and epidemic growth. In particular, due to the poor sensitivity and specificity of COVID-19 symptoms, and high incidence of non-COVID-19 COVID symptoms, tracing contacts of all those reporting these symptoms before test results are available will likely prove inefficient, placing a large demand on the system and potentially undermining its speed and effectiveness.
-
Maximizing population participation and compliance with TTI guidance. Keeping all those individuals found to be in contact chains engaged within the TTI system is also crucial to its success. Cases can leak from the TTI system at the point of initial symptom reporting (due to not having symptoms or choosing not to report them), and at every stage thereafter, including timely testing, contact finding and compliance with requests/requirements to test, quarantine or isolate. As a result, the impact of adding TTI on transmission is sensitive to the ability of the system to reach affected individuals and such individuals’ ability to comply with guidance. Some of these leakages can be addressed through improved TTI systems, including potentially the use of phone-based apps. However, incentives to participate and comply are also likely to be needed. Insights from other health and non-health work suggest several options for creating such incentives, including incentives for app use (e.g., more rapid testing, improved access to health advice and support during quarantine and isolation) and working with employers and communities to incentivize groups through benefits for all (e.g., access to relaxed NPI regimes).
-
Increasing epidemic coverage of TTI. Bringing more contact chains within the ambit of TTI is central to its effectiveness. Increasing TTI coverage requires strong data collection and management systems, and the integration of case-based data with broader surveillance efforts, particularly for capturing new chains as they flare up. In this respect, surveillance and TTI are mutually beneficial, since TTI can capture important data on index cases and contacts, while surveillance can provide indications of who/where to target for testing, even when they are not part of an existing identified chain. This paper proposes ways in which the UK might design an efficient surveillance process and analyse the data collected to maximize the effectiveness of TTI. Existing data collection efforts - including those from TTI, from other ad-hoc systems (e.g., self-reported symptoms or calls to health actors) and from systematic population-based testing work such as the ONS COVID-19 Infection survey – need to be coordinated to ensure standardized data collection that will allow triangulation of information and localization of responses. This requires increased granularity of data in terms of person, time and place for new transmission chains, outbreaks and each individual case and contact.
-
Managing TTI capacity constraints. As the TTI system becomes faster and more comprehensive, tracing and testing capacity may be reached, which will in turn slow testing and tracing, reducing the benefits of the system. While in the long-term TTI capacity may be expandable, if capacity limits are reached in the short-term, important decisions will need to be made about how to prioritize within TTI. These choices can be made on at least two key axes: prioritizing index case and contacts on the basis of risk; and acting based either on index case symptoms or test results. In particular, in our model assuming moderate NPIs, tracing and quarantining contacts based on index case symptoms as opposed to a positive test led to a five-fold increase in the number of tracing events required (to as many as 1 million per day), and a 52-86% increase in the number of quarantine days required. All the successful TTI programs worldwide that we reviewed based contact tracing on laboratory confirmed index cases. To manage all these choices, a clear dynamic strategy will be needed for managing TTI capacity as the epidemic changes size and location. Strong integration of information from within TTI with wider surveillance and electronic health records will help improve the decision process (e.g., by predicting likely positive infections in index cases and contacts).
-
Effective TTI within a broader epidemic response. TTI is most effective when it is comprehensive and efficient, rapidly reaching and ending most infection chains. However, even in the best of circumstances, TTI will not capture all transmissions, especially for COVID-19 due to asymptomatic and mild cases. Our review of existing literature and our own modelling exercise strongly suggest that TTI can offer some assistance to the COVID-19 epidemic response, provided that it is well-integrated with other complementary, population-based NPIs and intensive outbreak investigations in settings where the force of infection is high and widespread (e.g., care homes, hospitals, hostels). We highlight the importance of considering incidence levels of illnesses with similar symptoms and the capacity of the system itself to manage caseloads; similarly, deciding when to adjust NPIs will be informed by surveillance as well as the capacity of the local TTI system itself. Effective TTI will require substantial coordination across a wide range of organizations, including central and local government departments, Public Health England, the NHS and business groups. Integration of TTI and surveillance at local levels would create important sources of data to support decision making for, and potentially provide synergies for implementation of, locally differentiated outbreak management. As the national epidemic declines, TTI will be play an increasingly important role in ending chains of transmission arising from distinct outbreak events and preventing re-emergence of generalized community transmission.
Test, trace, isolate
1. The role of TTI in COVID-19 response
In early May 2020, the UK was estimated to be experiencing 10-20,000 incident cases of COVID-19 per day. The UK’s focus in the short- to medium-term appears likely to be on containing the COVID-19 epidemic and driving down case numbers, while balancing policies to reduce transmission alongside policies to manage the wider health and economic impacts of the epidemic. Achieving this balance will require the dynamic balancing of non-pharmaceutical interventions (NPI) as the epidemic size fluctuates. Testing, tracing and isolation (TTI) is a tool that helps to identify and diagnose cases, reduce onward infections, and provide vital information that can support decision making on policy balance. TTI’s overall effectiveness and ability to contribute to epidemic control is a function of policy choices regarding the TTI system’s capacity, optimisation and support for compliance, alongside wider choices about surveillance and other NPI measures.
All aspects of a coordinated TTI system must be firmly guided by the core public health purpose of reducing transmissions and contributing to maintaining an effective reproduction number ($R_e$) below 1. Alongside its public health benefits, the system enables identification of cases for clinical care and provides intelligence on the course of the epidemic (surveillance), which in turn enables TTI to be targeted to optimise its primary purpose.
This report combines data and ideas relating to TTI from several sources, including an empirical review of the implementation and impact of TTI in the context of COVID-19, reviews of the potential role of the business sector and of systematic surveillance in supporting TTI, and a single-generation mathematical model to capture how variation in TTI implementation might affect the $R_e$. The report’s overall goal is to present evidence on how TTI might be implemented and what its impact might be as part of a COVID-19 epidemic response.
COVID-19, like any infectious disease epidemic, is a collection of transmission chains. A core principle of epidemic control for infectious disease lies in preventing onward transmission by breaking these chains through reducing interactions between infectious index cases and susceptible contacts. This requires interventions to identify infected individuals and quarantine their contacts before they themselves become infectious.
TTI is the chained process of testing individuals with symptoms or known contact with past cases (index individuals), and then tracing and monitoring contacts of these index cases and potentially limiting their interactions (through supported isolation or quarantine1). TTI reduces the speed at which the epidemic grows by identifying those at greatest risk of having been infected and separating them from the general population. Contact tracing can allow infected contacts to be identified while they are still incubating the infection (and thus not able to transmit) and while asymptomatically infectious (curtailing any onward transmissions). A combination of testing, tracing and isolation can therefore be potentially powerful in controlling COVID-19. In this report, we consider TTI as reactive, starting when index cases self-reports symptoms, as opposed to proactive population testing (potentially based on risk factors), although we do discuss how the two might be integrated and support one another.
TTI is typically applied in the context of other actions that can reduce the number of potential infectious interactions in the population. These actions fall roughly into two categories: those reducing the chance of infection per contact, and those reducing the number of contacts. Measures that reduce the chances that an interaction leads to transmission include infection control in institutional settings where social distancing may not be possible (e.g., hospitals, care homes, prisons), increased hand washing, respiratory hygiene and use of masks and other personal protective equipment. Measures that reduce the number of contacts between all individuals in the population include social distancing (e.g., working from home, avoiding meetings with friends and family), bans on mass gatherings and closure of schools, shops and restaurants. Importantly, the latter greatly reduce inter-household and community transmission, but do not much affect intra-household transmission, a major source of secondary infections. Contact tracing can therefore be seen as complementary to social distancing and infection control in the context of COVID-19, insofar as it is able to either drive within-household infection prevention efforts (i.e., self-isolation), or provide a gateway to extra-household isolation if required.
Countries that have managed to, at least temporarily, control their COVID-19 epidemics have almost all enacted and maintained substantial testing and contact tracing efforts from early in their epidemics. These countries include China, Iceland, New Zealand, Singapore, South Korea and Taiwan (see summary in Technical Document 4). There are several commonalities in these countries’ TTI approaches:
-
Comprehensive TTI was started early in the epidemic, when the number of contacts needing tracing, and thus of follow-on tests, were small;
-
Testing provision was widespread, decentralised and accessible in both traditional and novel locations, including primary healthcare settings;
-
Turnaround times from symptom reporting to test result provision were short. For example, it takes around 5 hours from swab collection to test result provision in Vietnam2 as a result of the government ramping up laboratory analysis capacity. Similarly, in Taiwan3, tests results can be provided in 4 hours and in South Korea4, tests results are provided through automated text messages within 24-48 hours of swab collection.
-
Contact tracing was conducted rapidly, ensuring contacts were reached before they became infectious. South Korea, for instance, uses an information communications technology (ICT) system that integrates GPS data, credit card information and CCTV footage to create a moving history (i.e. transmission route) of the confirmed case in 10 minutes, which is matched with the patient interview, and contacts are then identified and informed via text messages on the same day. The whole process of testing, contact tracing and isolation advice for contacts takes approximately 2-3 days in South Korea.
-
Compliance with isolation was high due either to tailoring to homes (where physical separation was feasible) or through physical separation in institutions (e.g., hotels) or enforcement through fines on violation (South Korea and Taiwan)56;
-
Traditional manual tracing strategies were supplemented with app-based approaches for efficiently notifying contacts of cases and conducting follow-up symptom checks.
However, it is not yet possible to quantify the independent effect TTI has on COVID-19 epidemic control, since these countries generally also had strong early social distancing and infection control procedures.
2. Factors that influence optimal performance of a TTI system
COVID-19 presents a particularly challenging disease for control via TTI because of its:
-
Short serial interval (time between an index case and infected contacted becoming infectious);
-
High level of pre-symptomatic transmissions7 (those occurring prior to symptom presentation);
-
Non-specific and often mild symptoms; and
-
Sometimes subclinical presentation (leading to asymptomatic transmissions).
These challenges mean that three aspects of a TTI system will prove crucial to its effectiveness:
-
The speed at which testing and tracing contacts can be conducted at scale;
-
The level of compliance with guidance and participation in TTI; and
-
The coverage of new chains of transmission TTI, based on a wider surveillance system.
Several recent models have shown that while TTI help with epidemic control, a TTI system will need to be very efficient (little or no leakage,8 fast testing and tracing,9 comprehensive use of digital app-based contact tracing10) if used in isolation to have a substantive impact on a COVID-19 epidemic. This has led several teams have concluded that TTI will need to be part of a combined COVID-19 control strategy.11 12 13
Technical Document 3 presents a single-generation individual-based model, based on that of Kucharski et al., which explores the impact of pipeline speed and population compliance on the effectiveness and resource requirements of TTI over the coming summer in the UK. It considers three TTI strategies which trade off resource requirements and TTI pipeline speed: (i) initiating contact tracing on symptom presentation; (ii) initiating contact tracing only upon the index case testing positive; and (iii) each of these with additional testing of contacts. These strategies are evaluated in the context of a range of other government measures with varying stringencies, including app-based digital contact tracing. In all scenarios, we assume that on reporting symptoms, all possible index cases are requested to immediately isolate themselves and have their household contacts quarantine, pending test results.
Our understanding that the present intention in the UK is to include within the TTI system an app that combines symptom reporting with anonymized contact tracing and facilitates access to testing, but that most contact tracing will be done manually through PHE, with an as-yet unfinalised interface mechanism. Our model assumes a system that combines manual and app-based contact tracing and varies the level of app uptake. As a result, in our model an app can rapidly identify and inform contacts otherwise unknown to index cases, and speed quarantining of secondary contacts.
In Figure 1 we show results from our base case scenario. In this scenario we assume that contact tracing only commences after index cases test positive, that these testing and tracing processes take two and one day respectively, and that 80% of people will adhere to government guidelines on reporting symptoms, self-isolating and quarantining if told they are a contact of a case. When NPI are stringent (S5), most secondary infections are prevented by social distancing. In all scenarios, around 45% of the remaining infections are prevented by index case isolation and quarantining of household contacts. Adding contact tracing of extra-household contacts to the intervention package removes around 10% of the remaining infections; the rest are not prevented due to leakage in the TTI system, symptomatic individuals not reporting their illness and individuals who have mild or no symptoms and do not suspect they are infected.
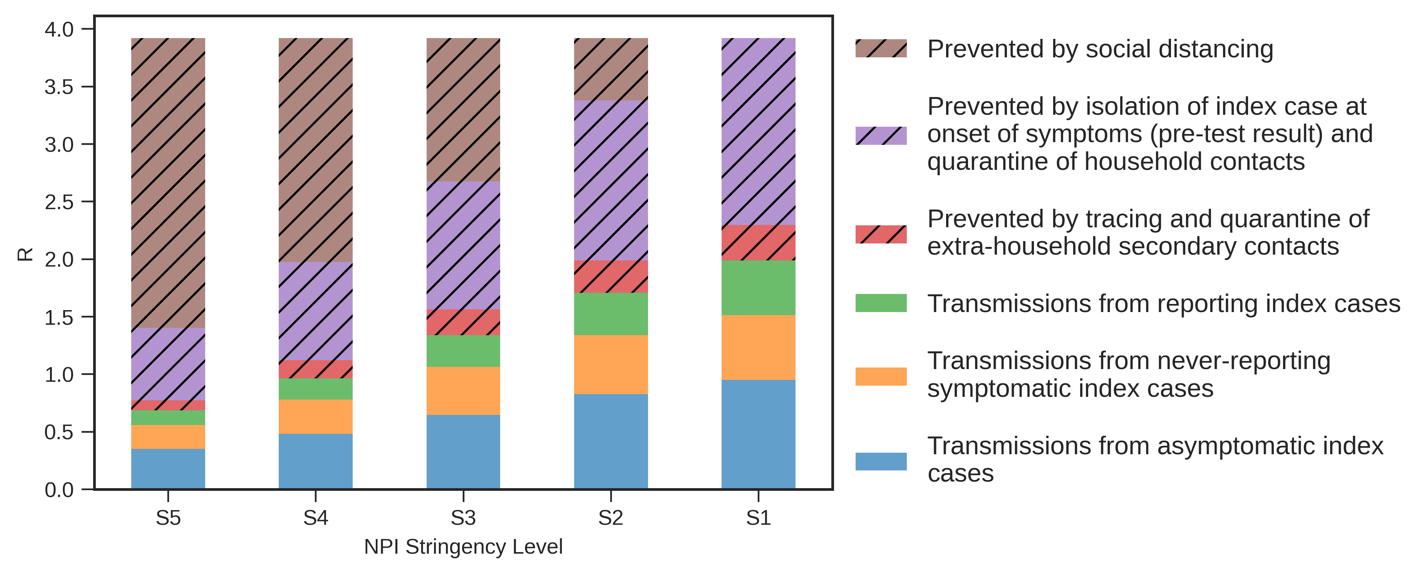
Figure 1. The contributions of different intervention strategies to preventing secondary Covid-19 infections.
S1 to S5 are increasingly stringent NPI scenarios. S1 involves no restrictions on social interaction, only requiring households of symptomatic individuals to quarantine; S5 reflects the situation before May 9th. $R_e$ reflects transmissions from index cases to their secondary and tertiary contacts. See Technical Document 3 for details.
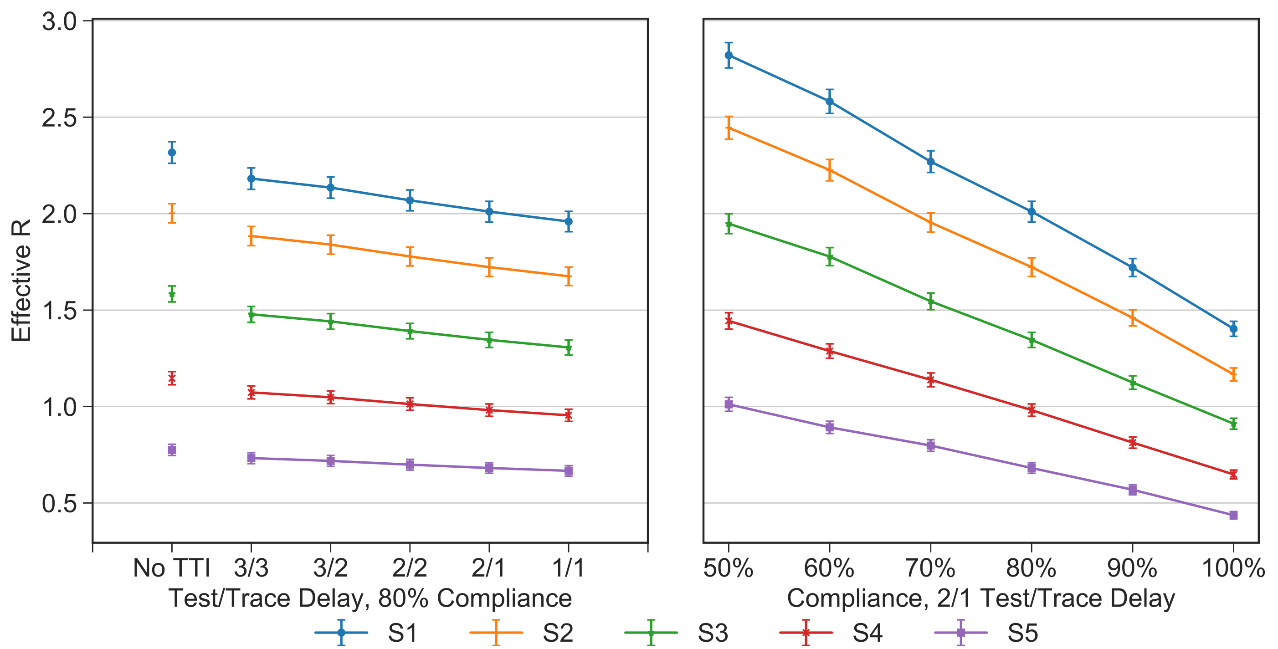
Figure 2. The impact of varying delays in testing index cases and tracing extra-household secondary contacts (Left). The impact of varying compliance with steps in the TTI system (Right).
The findings of our model highlight the importance of speed and compliance to the effectiveness of TTI. The figure above summarises the results for test-based TTI across five scenarios ranging from most (S5) to least (S1) severe NPI measures. Overall, this simulation indicates that contact tracing can generate a reduction in $R_e$ of 5-15%, depending on the stringency of other government measures and under reasonable assumptions about testing/tracing speed and public compliance.
3. Increasing speed of testing and tracing
The speed at which TTI can move along transmission chains is determined by two quantities: the time between an index case reporting symptoms and their test results being available, and the time required to trace their contacts. COVID-19 combines a short serial interval (5-6 days on average) and substantial pre-symptomatic transmission (up to 2 days prior to symptom presentation).14 This provides a short window within which infected contacts need to be reached by a TTI system to avoid onward transmission. Between January and March in the UK, the time taken to obtain a test result averaged 3 days, and the time to find a contact 2 days.15 This amounts to the entire pre-symptomatic period of the average secondary case, meaning that by the time contacts were found, half of their onward transmissions to tertiary cases had already occurred. Our simulation model finds that reducing the overall turnaround time from five days to three leads to 60% improvement in effectiveness of the test-based TTI system (in terms of reduction in $R_e$), due to the quarantining of infected contacts just as they are expected to be most infectious. For now, we assume that point-of-care testing is not imminent, although a high-quality, immediate test became available should provide a small additional benefit.
A key element of TTI effectiveness is system speed at breaking transmission chains. While the testing and tracing process can be sped up by asking secondary contacts to quarantine at the point where either their index case tests positive or even shows COVID-19 symptoms, these approaches have very large economic consequences in terms of days lost to quarantine (see section 6). Speeding up testing and tracing processes is thus vital. App-based contact tracing can reduce the time needed for manual tracing. However, the effectiveness of app-based tracing is determined by overall smartphone usage and willingness to install and use the app. Smartphones and app use may be lower among children, the elderly and lower socioeconomic status populations, there may be ongoing concerns around privacy and centralised vs decentralised tracing systems, and the population may uninstall the app as the epidemic wanes.
Some ways in which each delay in the system might be addressed are suggested below. Achieving substantial increases in speed is likely to require a system-wide approach utilising existing public health infrastructure and capacity, with greater linkage with primary and community care and local authorities. This decentralised approach is likely to enable faster test turnaround and improve on tracing delays.
-
Time from symptom onset to patient self-reporting. This can be helped by a well-designed, clear public information campaign on how and where to report symptoms which provide access appropriate to all (e.g., via the NHS COVID 19 App, NHS 111 or GP. Apps that also track symptoms may also be able speed up reporting, at least for a subsection of the population.
-
Time from symptom reporting to test taking place. This requires multiple access points and channels to testing, but any appointment systems must have minimal delays (at home or at a test centre). These systems could integrate the use of apps, as used recently for a chlamydia testing and risk assessment system.16 Self-swabs may increase speed but delivery would need to be rapid - one potential approach would be pick-up from central community points (e.g., pharmacies, community centres, GP hubs).
-
Time from testing to lab. Courier services are likely to be central here, but pharmacies as collation points for pick up within communities could also help, as might local NHS specimen collection.
-
Time to get test results in the lab. Optimisation is needed for specimen processing and RT-PCR speed, using agreed standard operating procedures (SOPs). Expansion of testing to any certified laboratory could also improve throughput.
-
Time to lab confirmation of results. Automation of results without the need for manual review using autoverification protocols is a possibility but timing and accuracy is of importance. Careful SOP use and automation may also improve quality assurance and minimise loss of tests and identifiers.
-
Time to return results to testee. Automation of results reporting by phone, email, app or text message have the potential to help here, if done with care include a secure method of verifying receipt that minimizes privacy concerns and includes simple and clear guidance on: (i) self-isolation household quarantine; (ii) preventing intra-household transmission; and (iii) the contact tracing process.
-
Time from positive result to contact tracing. Patient details, with consent, need to be concurrently passed to PHE as they are communicated to the testee. Once an app is live, some contacts will be automatically informed once index informed, and whether the information has been verified to be received by the recipient, but those without the app will still need to be managed manually. This manual PHE system will need to be linked with the app to know who has/has not been informed, especially if multiple testing channels are in place; this interface will be central. The creation of contact lists prior to testing generates logistical burdens and collects a lot of personal information that will ultimately not be used (since most people will test negative), which may reduce overall compliance.
4. Maximizing population participation and compliance with TTI guidance
While the mechanisms of the TTI system can be designed carefully to maximize speed and efficiency, its effectiveness relies centrally on the willingness of society to engage with it. There are multiple points in the TTI system where non-participation can occur:
-
Those with asymptomatic infections will not have symptoms to report,
-
Symptomatic cases may not self-report, due either to not noticing (subclinical infections) or concern regarding loss of earnings if required to self-isolate and social stigma if their contacts are required to quarantine;
-
Individuals may not complete a test if requested to complete one;
-
Tracing of at-risk contacts is likely to be incomplete, missing some important contacts and being unable to find others due to anonymity (e.g., on public transport) given the respiratory and fomite-related nature of transmission, desire to remain anonymous or due to non-cooperation of index cases;
-
Finally, identified contacts may not comply with requests to self-isolate on their own volition in their own homes (e.g., if this implies a loss of income), or not be willing or able to maintain infection control within their home (e.g., isolation in a separate bedroom, bathroom and cutlery, mask use).
As a result, the potential for transmissions and infectious individuals to escape the TTI process is substantial.
In our simulation study, we used a single compliance parameter to represent general compliance with government guidelines, specifically it refers to the proportion of the population who will: (i) report symptoms when they get them; (ii) quarantine their households; and (iii) self-isolate when instructed to when contact-traced from an index case. We found that the level of compliance significantly affects our results. For example, for test-based TTI in the context of moderate NPIs, $R_e$ falls from 1.9 at 50% compliance to 1.4 at 80% compliance. We considered the added benefit of app-based tracing, but found it to have a relatively minor impact on effectiveness in our base-case scenario. This reflected our assumptions that all contacts of each index case will still be (more slowly) manually traced in addition to any app-based tracing (assuming we will not be able to tell which of a person’s contacts have been traced via the app), and an optimistic one-day turnaround time to carry out manual tracing. Since app-based tracing is effectively instantaneous, the longer manual tracing takes, the greater the benefits of the app.
Maximizing population participation and compliance will necessarily draw on psychological and economic, as well as technological, factors. Approaches will need to consider two key factors:
Avoid Perception of Negative Consequences from Engaging in TTI
People are less likely to engage in the TTI process if they believe that this will lead to a substantial burden in terms of testing, quarantine or isolation for themselves or others. An important consideration when considering whether to quarantine contacts based on index case symptoms (rather than a positive test) is how the resulting unpopularity with family and work colleagues - particularly if they ultimately test negative for SARS-COV-2 - will affect the willingness of index cases to report those symptoms. The speed benefits of quarantining contacts at the point that index cases report symptoms may be outweighed by decreased TTI engagement.
Another important consideration in the context of integrating a tracking app into the TTI process is the level of public confidence engendered by a centralized app system. Independent of the actual risks of data integrity, perceptions of the possibility of data being lost or misused will affect willingness to use the app or become involved in the TTI process more broadly. These uptake costs will need to be considered in light of the technological benefits of a centralized system, and careful public messaging will be required to reassure the public that the risks are reasonable given the benefits.
Incentivize Participation in TTI
As well as minimizing avoidable losses, steps can be taken to actively increase involvement in the TTI process. These processes will need to be integrated across sectors of society and include pro-social messaging, incentives and potentially sanctions for non-involvement.
Clear messaging is needed on how to participate in TTI, and what the benefits of participation are at both the individual and population level. Messaging to the public can emphasize that involvement in TTI will provide population-level benefits, by reducing epidemic spread, and potentially allowing greater freedoms. Making messaging effective involves more than providing credible information, it relies on creating a consistent and appealing narrative for TTI. One area where this will be crucial is for any tracking or tracing app - since its usefulness scales quadratically with uptake (both index and contact need to have the app for it work). Harnessing pre-existing social networks in messaging can enhance trust in the system. Thus, to be effective, such dissemination should use local networks where trust is highest, such as GP surgeries, clubs, community groups, unions and professional associations and religious organizations.
However, evidence from a range of public programs shows that appeals to individuals’ pro-social motivation, especially when they are asked to take actions which are personally costly, will be insufficient alone: effective deployment of TTI will therefore also require consideration of incentives and sanctions. Evidence from RCTs in developing countries have shown that even small incentives can enhance compliance with testing for HIV, partly by increasing the salience of the benefits of such tests.17 TTI can be crafted to provide direct individual-level benefits through faster information provision. Willingness to participate in tracing can be linked to regular symptom checks, health advice and rapid access to testing and results; even more so if using an app. All this will need to be carefully designed to ensure provision of easy-to-follow, practical advice about how to comply with TTI guidance, including reporting procedures, testing processes and how to effectively self-isolate and practice infection control within the household.
Reaching beyond the individuals directly involved in TTI, there is also potential to incentivize employers and communities, as outlined in Technical Document 1. This will have benefits beyond TTI, for example when rolling out a vaccination program if that happens. Workplaces or geographies with high participation in testing, tracing, isolation or app use might be offered faster access to relaxed NPI regimes or workplace reopening; the extension of the furlough scheme to cover payments for isolation and quarantine could also incentivise staff compliance with testing and isolating as appropriate, among those who cannot work from home.
Whatever incentive structures are put in place, it will be important to consider their potential equity impacts, both for intrinsic reasons and since impact unequal effects are likely to suppress willingness to participate overall. Notably, some groups at greatest risk of COVID-19 acquisition and poor outcomes (e.g., the elderly, black, Asian, and minority ethnic communities,18 frontline service-sector workers19) may be less likely to have smartphone access, and those industries and groups most affected by NPIs (e.g., the self-employed) may be least easily engaged in workplace programs.
5. Increasing epidemic coverage of TTI
The existence of pre-symptomatic, and particularly asymptomatic or symptom non-specific, transmission for COVID-19 may generate a substantial number of transmission chains that will not be picked up even by a highly efficient TTI system. Beyond the mechanics of the TTI process, there are several ways in which the use of TTI can be targeted to further increase efficiency. These revolve around maximizing the ability to identify those most likely to be infected. Strategies to achieve this include continuously improving predictive models as knowledge of more specific symptoms of COVID-19, such as loss of smell, comes to light, and prioritising individuals likely to have greatest potential transmission to others (e.g., through their occupation or geographical localisation). Provided testing protocols are consistent, TTI test results can be used to augment survey data and so refine local estimates of incidence.
Finding people infected with COVID-19 when community transmission is common will require a detailed understanding of how incidence, prevalence and risk factors for COVID-19 vary across geography, demography and economic sectors. Such understanding will in turn, as outlined in Technical Document 2, require well-designed country-wide stratified quasi-random sampling and diagnostic testing, which can be triangulated with self-reported symptom data (e.g., through apps such as the Zoe COVID Symptom Study app) and case reporting data.
This triangulated data will provide real-time, stratum-specific incidence predictions to inform selection of individuals for TTI follow-up, as well as sample adaptation for subsequent surveillance. Such surveillance data can help target initial testing procedures at those likely to become infected (e.g., young people) and those likely to be infectious (e.g., service staff in busy establishments, especially health and social care settings). They can also help target tracing and secondary testing efforts (e.g., focusing on household contacts, those still working). A fuller understanding of the geographic spread of COVID-19 could also allow focused use of scarce contact tracing resources to key areas of the country, especially as the epidemic grows smaller.
Technical Document 2 provides details on how the UK might design an efficient surveillance process and analyse the data collected to maximize the effectiveness of TTI, building on existing serological surveys such as that led by the Office for National Statistics.20 In addition to surveillance activities, business sectors could be profiled in a risk assessment exercise to determine the frequency with which testing would be required to reopen, with employers potentially responsible for compliance (see Technical Document 1). Expanding regular pro-active testing (and subsequent TTI as needed) to key economic sectors might also help to increase the overall proportion of transmission chains brought within the TTI system. Decisions to open sectors would need to be linked to how each business type might affect epidemic spread and its economic value.
Surveillance and TTI are mutually beneficial, since TTI can capture important data on index cases and contacts, while surveillance can provide indications of who/where to target for testing even when they are not part of an existing chain. For these synergies to be realised, however, data collection efforts need to be coordinated and asynchronous. Coordination is required through standardized data collection to allow triangulation across TTI, other health surveillance systems (e.g., calls to health actors or app-based symptom tracking) and systematic population-based testing. Maximum benefit will be gained when that surveillance systems provide information complementary to that arising from TTI, and additional to standardly available information sources. This combination of characteristics will allow surveillance to add benefit to TTI, helping to localize responses. Localisation could be by sector, for example to focus on particularly vulnerable sub-populations (e.g., the elderly), or by geography to take account of risk-factors for which small-area-level data are available (e.g., deprivation).
Standardized data collection processes will have to balance the need for key information with the data quality losses, and lack of participation, that may arise from asking for too much data. Core population-level data will need to include the number of newly infected individuals (including pre-symptomatic, asymptomatic and untested), stratified by geography, demography and sector. Prevalence of current (PCR) and past (antibody) infection can be estimated and tracked over time by stratified random population studies and case, hospital and mortality data. Added precision may be obtainable by supplementing “hard outcome” data of this kind with information from symptom tracker apps. At the individual level, key sociodemographic information will also need to be collected, including minimally age, sex, ethnicity, geographic location, household composition and occupation. An understanding of how index cases are connected to known transmission chains will hopefully be captured in the TTI process. All these data allow for targeting of limited resources, including testing algorithms, and planning for future resource needs and scenario feasibility.
6. Managing TTI capacity constraints
Building a faster, more comprehensive TTI system is central to it playing an effective role in combating the epidemic. Importantly, however, as tracing and testing capacity is reached, the speed of both is likely to reduce, which in turn reduces the benefits of the TTI system. While in the long-term TTI capacity may be expandable, in the shorter-term if capacity limits are reached, important decisions will need to be made about which activities will be prioritized.
Which contacts should be traced?
In an epidemic with highly sensitive and specific symptoms, it is more feasible to trace contacts of anyone presenting with symptoms. Since COVID-19 lacks specific symptoms (many are similar to a range of other common respiratory infections) and asymptomatic and pre-symptomatic cases are common, symptom-based tracing can become extremely resource intensive when epidemics are large or when other health conditions generate many false positives (e.g., seasonal influenza and colds). Furthermore, requiring many contacts of false-positive index cases to quarantine (if this is policy) will have substantial consequences for the economy, and could have limited public acceptability of TTI when contacts realise they have been needlessly isolated.
All the established TTI systems we reviewed (Technical Document 4) trace only the contacts of index cases who have tested positive for COVID-19, as opposed to all those reporting symptoms. While test-based tracing introduces a delay into the system that will generate some secondary cases, if capacity is limiting this may be balanced by a faster contact tracing process once begun, due to the reduced number of tracing events required, and reduced resource requirements. For example, daily incident COVID infections in mid-May were estimated to be 10,000 (we used an upper confidence bound of 20,000 in our simulations),21 while daily pre-COVID fever or cough presentations over the summer averaged 100,000,22 23 and the average number of close contacts that would have to be quarantined is on the order of 10-12 per index case.24 25 26 Our simulations found that a symptom-based tracing system generated 52-86% more person-days of quarantine and over five times as many manual traces than a test-based one – on the order of 1 million contacts needing manual tracing each day, over 80% of whom will later prove unnecessary when the index case tests negative. While absolute numbers here are driven by seasonality and epidemic stage, the relative resource requirement of symptom-based testing remains substantially higher across a reasonable range of scenarios.
An intermediate position between a fully-symptom and fully-test-positive approach could be to augment symptoms with other information about index cases (e.g., risk factors for transmission such as occupation) to decide whether or not to immediately start contact tracing. As TTI system capacity improves, or the epidemic wanes, contact tracing could be expanded to an ever-wider set of symptom-positive index cases. If a surveillance system is in place, it can be used to increase specificity of symptom-based contact tracing to target (e.g., local outbreaks) and lowering risks to vulnerable populations (e.g., in care homes and primary care).
Beyond selectively choosing to trace index cases’ contacts as a whole, there is the potential within TTI to choose which contacts to trace. It is well established that household and other repeated, close or prolonged contacts (e.g., work colleagues) are at greater risk of acquisition27 28 29 30 31 32 and might therefore usefully be prioritized. Similarly, the tracing effort required to find one-off, relatively brief contacts unknown to the index case is likely to be greater, suggesting that when capacity is constrained a focus on well-known contacts may be an efficient choice. Again, this provides an opportunity to use data gathered from other sources, including household contact studies, alongside the wealth of TTI information on secondary infection yield by contact type to target contact tracing if necessary. Real-time analysis integrating data from pre-existing sources, case/TTI systems and designed incidence studies to produce stratum-specific predictive probability maps of incidence can inform selection of individuals for TTI follow-up, potentially through risk assessments on app or triage systems that are dynamically adjusted by epidemic and NPI levels. These predictive maps can also be used to adapt future sampling strategies where pro-active TTI is being conducted, to maximise efficiency and coverage.
Which contacts should be tested, and when?
A key question in the context of TTI is whether to test contacts, and if so when. In our modelling we showed that, depending on the severity of the other NPIs in place, testing all contacts of index cases who test positive would require 50-171% more tests than using symptoms to guide such tests, but without a substantial impact on $R_e$ (since given testing and tracing delays, the only people they differentially capture are those who are asymptomatic). In situations where all contacts of positive index cases are advised to quarantine for 14 days, even though policies typically require testing only occur after symptom onset, most onward transmission will be strongly limited by the quarantine process. However, as noted above, this has a substantial social and economic impact on quarantined individuals and requires ongoing monitoring effort from the TTI system. When capacity is limited, selective testing of contacts may allow them to be released from quarantine (and thus from active symptom surveillance) earlier - as well as allowing the contacts of infected secondary contacts to be rapidly followed up. Prioritization decisions might again rely on the likelihood of having acquired infection, but might also want to take account of the opportunity cost of being quarantined (e.g., focus on working-age adults). It is, however, vital not to test too early in the infection process (since RT-PCR tests are insensitive during much of the incubation period,33 or to consider repeated testing after a period equivalent to the presumed incubation period, before releasing people from quarantine. Optimization of testing strategies will therefore require a clear understanding of likely transmission timing.
Which contacts should be asked to isolate, and when?
The ‘isolate’ part of the TTI system also raises the potential for trade-offs. The most comprehensive approach would be to quarantine contacts based on symptoms in the index case, as soon as they are found. If capacity is limiting, the very large number of people under TTI monitoring may overwhelm capacity, at least in the short term. Once more, these numbers can be reduced either by changing the criteria based on the index case (so isolating contacts based on the index case testing positive instead of based on symptoms), or by selecting those contacts at greatest risk of being infected or generating onward transmissions. This balance can again be calibrated depending on capacity constraints and epidemic stage.
7. Effective TTI within a broader epidemic response
The effectiveness of contact tracing is highest when the information picture of contact chains (through surveillance and reporting) is robust, when TTI capacity can contain almost all known contact chains, and speed, efficiency and compliance is high. With a growing epidemic, as the capacity to infection ratio declines, so do the benefits of TTI. In the midst of a large outbreak with substantial community spread, TTI is likely to be resource-intensive, while providing a relatively small benefit due to slow turnaround times. Nevertheless, under the expectation that the UK is likely in the short- to medium-term to focus on containing the COVID-19 epidemic and driving down case numbers, TTI can play an important role. The exact nature of this role will depend on the resources available to it, and the policy decisions made regarding how it fits within the wider epidemic response. We have outlined some of the possible TTI activity choices and explored their likely impacts.
Several of these decisions relate to a careful balance of the health and economic impacts of the epidemic. Central to such a balancing act are careful decisions about when to adjust NPIs. TTI provides a policy tool that may allow somewhat greater relaxation of NPIs than would otherwise be possible, thus the resources required to conduct TTI may provide benefits in terms of reduced NPI stringency and thus greater economic productivity. However, TTI’s benefits will depend on how it is implemented. As highlighted above, TTI is most effective when it is able to be comprehensive and efficient, rapidly reaching and ending most infection chains. It is likely to be particularly important when considering relaxing NPIs, e.g., when the number of new cases (in a given area/sector) is declining. Deciding when to adjust NPIs will be best informed by surveillance as well as the capacity of TTI itself.
Importantly, contact tracing approaches alone are unlikely to be efficient in settings with very high force of infection (e.g., care homes, hospitals) since tracing is most useful when some people are at substantially greater acquisition risk than the general population - although TTI for families and other close contacts of healthcare staff will still be important. In high-risk settings, outbreak investigation and infection control approaches are needed, to identify both who has been infected (likely widely dispersed across the institution) and where infection control has failed allowing the initial and secondary infections to arise.
Based on all of the above, we believe that TTI will require a joined-up epidemic response, both to maximize its internal efficiency and its effectiveness in support of other work. Effective TTI will benefit social distancing and other contact-reducing interventions by reducing the number of potentially infectious contacts through increasing infected individuals’ status awareness and their willingness to isolate. Effective TTI can also bolster the effectiveness of antiviral treatments (once available), by getting infected individuals onto treatment early in their infection, rapidly controlling their viral load.
Nevertheless, effective TTI will require substantial coordination across a wide range of organizations. This will include public health bodies such as Public Health England, healthcare bodies such as the NHS and private healthcare (as well as NHSx), central government departments covering healthcare, finance and business, and local authorities who can potentially play a vital role coordinating activities as the response become localized in response to epidemic heterogeneity. For example, strong coordination between app-based tracing (managed by NHSx) and manual tracing (managed by PHE) will be needed to avoid duplication of work and to reduce the pressure on manual tracing. Finally, integration of TTI and surveillance at local levels would, in particular, create important sources of data to support decision making for, and potentially provide synergies for implementation of, locally differentiated outbreak management.
8. Next steps
This report, and its technical addenda, were necessarily limited by the time-sensitive nature of the request for its generation for input into active policy decisions. There are several important ways in which this work could be extended. These could include:
-
Addition of cost-effectiveness analysis to weigh up the lost productivity of quarantine versus health and healthcare costs;
-
Consideration of the trade-offs inherent in tight or loose criteria for contact tracing;
-
Consideration of the impact of waiting for different triggers to quarantine or isolate contacts;
-
Addition of more robust data for parameter estimation;
-
Explicit consideration of how surveillance and TTI might interact, and be used to conduct to proactive and risk-driven TTI, in a modelling framework;
-
Consideration of how TTI might reduce morbidity and mortality through earlier detection impacting clinical management; and
-
Extension of the current single-generation model to a dynamic longer-term model;
-
Evaluation of how findings differ in the context of substantially smaller epidemic states.
Many of these points highlight the need to include ongoing evaluation of the TTI system as it is rolled out and the epidemic evolves.
Technical Appendices
The following are materials prepared by individual members of DELVE as inputs into this report.
- TTI-TD1: The Potential Role of Firms in Test, Trace, Isolate
Prepared for the DELVE Initiative by
- TTI-TD2: Surveillance for Test, Trace, Isolate
Prepared for the DELVE Initiative by
- TTI-TD3: Effectiveness and Resource Requirements of Test Trace Isolate Strategies
Prepared for the DELVE Initiative by
- TTI-TD4: A Review of International Approaches to Test, Trace, Isolate
Prepared for the DELVE Initiative by
Simulation Software
Footnotes and References
-
Isolation refers to the separation of infected individuals from uninfected individuals, while quarantine refers to the separation of individuals exposed to infection in order to determine whether they have contracted a disease. ↩
-
Thai PQ., (2020). Addressing COVID-19 in Vietnam: Progress to date and future priorities. Usher Institute Webinars. 13 May 2020, https://www.youtube.com/watch?v=wibA34w8Xyo ↩
-
Lin, C., Braund, W., Auerbach, J., Chou, J. H., Teng, J. H., Tu, P. and Mullen, J. (2020), Policy Decisions and Use of Information Technology to Fight 2019 Novel Coronavirus Disease, Taiwan. Emerging Infectious Diseases, Vol. 26. (doi: 10.3201/eid2607.200574) ↩
-
The Government of Republic of Korea (2020). How Korea responded to a pandemic using ICT Flattening the curve on COVID-19. Published online April 15. Available at: http://www.moj.go.kr/moj_eng/1765/subview.do;jsessionid=OxKrsTASqh8vttNtuzZN_O7ALY7MJKI8vWSrtLao.wizard-10-25vxq?enc=Zm5jdDF8QEB8JTJGYmJzJTJGbW9qX2VuZyUyRjUxJTJGNTIyODIyJTJGYXJ0Y2xWaWV3LmRvJTNG ↩
-
Normile, D. (2020) Coronavirus cases have dropped sharply in South Korea. What’s the secret to its success? 17 March 2020. Science. Available at: https://www.sciencemag.org/news/2020/03/coronavirus-cases-have-dropped-sharply-south-korea-whats-secret-its-success ↩
-
Sheng-Iun, H. et al. (2020) New Taipei City man fined, taken to quarantine center. Taipei Times, 1 April 2020. Available at: https://www.taipeitimes.com/News/taiwan/archives/2020/04/01/2003733788 ↩
-
Cheng H, Jian S, Liu D, Ng, T., and Huang, W., Lin, H. (2020) Contact Tracing Assessment of COVID-19 Transmission Dynamics in Taiwan and Risk at Different Exposure Periods Before and After Symptom Onset. JAMA Intern Med. Published online May 01, (doi:10.1001/jamainternmed.2020.2020) ↩
-
Hellewell, J., Abbott, S., Gimma, A., Bosse, N., Jarvis, C., Russell, T., Munday, J., Kucharski, A., Edmunds, WJ., Sun, F., Flasche, S., Quilty, B., Davies, N., Liu, Y., Clifford, S., Klepac, P., Jit, M., Diamond, C., Gibbs, H., van Zandvoort, K., Funk, S. and Eggo, R. (2020) Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts, The Lancet Global Health, Issue 4, 488-496 (doi:10.1016/S2214-109X(20)30074-7) ↩
-
Kretzschmar M., Rozhnova G., Bootsma M., van Boven, M., van de Wijgert J. and Bonten M. (2020) Time is of the essence: impact of delays on effectiveness of contact tracing for COVID-19. MedRxiv. https://www.medrxiv.org/content/10.1101/2020.05.09.20096289v1 ↩
-
Ferretti, L., Wymant, C., Kendall, M., Zhao, L., Nurtay, A., Abeler-Dörner, L., Parker, M., Bonsall, D. and Fraser, C. (2020) Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing, Science (doi:10.1126/science.abb6936) ↩
-
Kretzschmar, M., Rozhnova, G., and van Boven, M. (2020) Isolation and Contact Tracing Can Tip the Scale To Containment of COVID-19 In Populations with Social Distancing. PrePrint: The Lancet (doi: 10.2139/ssrn.3562458) ↩
-
Kucharski, A., Klepac, P., Conlan, A., Kissler, S., Tang, M., Fry, H., Gog, J., Edmunds, J. and CMMID COVID-19 Working Group (2020) Effectiveness of isolation, testing, contact tracing and physical distancing on reducing transmission of SARS-CoV-2 in different settings, medRxiv 2020.04.23.20077024 (doi: 10.1101/2020.04.23.20077024) ↩
-
Goscé, L., Phillips, A., Spinola, P., Gupta, R.K., and Abubakar, I. (2020) Modelling SARS-COV2 Spread in London: Approaches to Lift the Lockdown. Preprints 2020, 2020050055 ↩
-
He, X., Lau, E., Wu, P., Deng, X., Wang, J., Hao, X., Lau Y et al. (2020) Temporal dynamics in viral shedding and transmissibility of COVID-19. Nature Medicine: 26, 672-675 (doi: 10.1038/s41591-020-0869-5) ↩
-
PHE, personal communications ↩
-
Estcourt C., Gibbs J, Sutcliffe L., et al. (2017) The eSexual Health Clinic system for management, prevention, and control of sexually transmitted infections: exploratory studies in people testing for Chlamydia trachomatis. Lancet Public Health, 2(4): e182‐e190. (doi:10.1016/S2468-2667(17)30034-8) ↩
-
Kranzer, K., Simms, V., Bandason, T., Dauya, E., McHugh, G., Munyati,, S, Chonzi, P., Dakshina, S., Mujuru, H., Weiss, H., Ferrand, R. (2018) Economic incentives for HIV testing by adolescents in Zimbabwe: a randomised controlled trial. Lancet HIV, 5, 2, e79-e86. (doi: 10.1016/S2352-3018(17)30176-5) ↩
-
Aldridge, R.W., Lewer, D., Katikireddi, S.V., Mathur, R., Pathak, N., Burns, R., Fragaszy, E.B., Johnson, A.M., Devakumar, D., Abubakar I, Hayward, A., 2020. Black, Asian and Minority Ethnic groups in England are at increased risk of death from COVID-19: indirect standardisation of NHS mortality data. Wellcome Open Research, 5: 88 (doi: 10.12688/wellcomeopenres.15922.1) ↩
-
Lan, F., Wei, C., Hsu, Y., Christiani, D. and Kales, S. (2020) Work-related Covid-19 transmission. medRxiv, Available at: https://www.medrxiv.org/content/10.1101/2020.04.08.20058297v2 ↩
-
Office for National Statistics (2020) Coronavirus (COVID-19) Infection Survey pilot: England, 10 May 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/coronaviruscovid19infectionsurvey/england10may2020 ↩
-
Flaxman, S., Mishra, S., Gandy, A., Unwin, H., Coupland, H., Mellan, T., Zhu, H., Berah, T., Eaton, J., Perez Guzman, P. and Schmit, N. et al. (2020) Report 13: Estimating the number of infections and the impact of non-pharmaceutical interventions on COVID-19 in 11 European countries. Available at: https://spiral.imperial.ac.uk/handle/10044/1/77731
(doi: 10.25561/77731) ↩ -
Smith, C.M., Conolly, A., Fuller, C., et al. (2019). Symptom reporting, healthcare-seeking behaviour and antibiotic use for common infections: protocol for Bug Watch, a prospective community cohort study. BMJ Open, 9, 5 (doi: 10.1136/bmjopen-2018-028676) ↩
-
Cowling, Benjamin J., et al (2020) Impact assessment of non-pharmaceutical interventions against coronavirus disease 2019 and influenza in Hong Kong: an observational study. The Lancet Public Health (doi: 10.1016/S2468-2667(20)30090-6) ↩
-
PHE, personal communications. ↩
-
Klepac, P., Kissler, S. and Gog J. (2018) Contagion! The BBC Four Pandemic - The model behind the documentary., Epidemics, 24, 49 - 59 (doi: 10.1016/j.epidem.2018.03.003) ↩
-
Mossong, J., Hens, N., Jit, M., Beutels, P., Auranen, K., Mikolajczyk, R., Massari, M. et al. (2008) Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS medicine, 5, 3. (doi: 10.1371/journal.pmed.0050074) ↩
-
Bi, Q., Wu, Y., Mei, S., Ye, C., Zou, X., Zhang Z. et al. (2020) Epidemiology and Transmission of COVID-19 in Shenzhen China: Analysis of 391 cases and 1,286 of their close contacts: a retrospective cohort study. Lancet Infectious Diseases. 2020 (doi:10.1016/S1473-3099(20)30287-5) ↩
-
Burke R., Midgley C., Dratch, A., Fenstersheib, M., Haupt, T., Holshue, M., et al. (2020) Active Monitoring of Persons Exposed to Patients with Confirmed COVID-19 — United States, January–February 2020. MMWR Morb Mortal Wkly Rep. 6 March 2020, 69, 9, 245–6 (doi: 10.15585/mmwr.mm6909e1) ↩
-
Luo, L., Liu, D., Liao, X., Wu, X., Jing, Q., Zheng, J., et al. (2020) Modes of contact and risk of transmission in COVID-19 among close contacts, MedRxiv, (doi: 10.1101/2020.03.24.20042606) ↩
-
Cheng, H., Jian, S., Liu D., Ng T., and Huang W. (2020) High transmissibility of COVID-19 near symptom onset. MedRxiv http://medrxiv.org/lookup/ (doi: 10.1101/2020.03.18.20034561) ↩
-
Chen, Y., Wang, A., Yi, B., Ding, K., Wang, H., Wang, J., et al. (2020) The epidemiological characteristics of infection in close contacts of COVID-19 in Ningbo city. Chin J Epidemiol. 41 ↩
-
Jing, Q-L., Liu, M-J., Yuan, J., Zhang, Z-B., Zhang, A-R., Dean, NE., et al. (2020) Household Secondary Attack Rate of COVID-19 and Associated Determinants. MedRxiv. (doi: 10.1101/2020.04.11.20056010) ↩
-
Kucirka LM, Lauer SA, Laeyedecker O, Doon D, Lessler J Variation in False-Negative Rate of Reverse Transcriptase Polymerase Chain Reaction–Based SARS-CoV-2 Tests by Time Since Exposure. Annals of Internal Medicine. 2020. (doi: 10.7326/M20-1495) ↩